Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial
- PMID: 35065057
- PMCID: PMC8840977
- DOI: 10.1016/S2468-1253(21)00427-1
Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial
Abstract
Background: Hepatocellular carcinoma has high recurrence rates after surgery; however, there are no approved standard-of-care neoadjuvant or adjuvant therapies. Immunotherapy has been shown to improve survival in advanced hepatocellular carcinoma; we therefore aimed to evaluate the safety and tolerability of perioperative immunotherapy in resectable hepatocellular carcinoma.
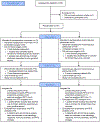
Methods: In this single-centre, randomised, open-label, phase 2 trial, patients with resectable hepatocellular carcinoma were randomly assigned (1:1) to receive 240 mg of nivolumab intravenously every 2 weeks (for up to three doses before surgery at 6 weeks) followed in the adjuvant phase by 480 mg of nivolumab intravenously every 4 weeks for 2 years, or 240 mg of nivolumab intravenously every 2 weeks (for up to three doses before surgery) plus one dose of 1 mg/kg of ipilimumab intravenously concurrently with the first preoperative dose of nivolumab, followed in the adjuvant phase by 480 mg of nivolumab intravenously every 4 weeks for up to 2 years plus 1 mg/kg of ipilimumab intravenously every 6 weeks for up to four cycles. Patients were randomly assigned to the treatment groups by use of block randomisation with a random block size. The primary endpoint was the safety and tolerability of nivolumab with or without ipilimumab. Secondary endpoints were the proportion of patients with an overall response, time to progression, and progression-free survival. This trial is registered with ClinicalTrials.gov (NCT03222076) and is completed.
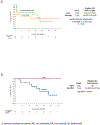
Findings: Between Oct 30, 2017, and Dec 3, 2019, 30 patients were enrolled and 27 were randomly assigned: 13 to nivolumab and 14 to nivolumab plus ipilimumab. Grade 3-4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The most common treatment-related adverse events of any grade were increased alanine aminotransferase (three [23%] of 13 patients on nivolumab vs seven [50%] of 14 patients on nivolumab plus ipilimumab) and increased aspartate aminotransferase (three [23%] vs seven [50%]). No patients in either group had their surgery delayed due to grade 3 or worse adverse events. Seven of 27 patients had surgical cancellations, but none was due to treatment-related adverse events. Estimated median progression-free survival was 9·4 months (95% CI 1·47-not estimable [NE]) with nivolumab and 19·53 months (2·33-NE) with nivolumab plus ipilimumab (hazard ratio [HR] 0·99, 95% CI 0·31-2·54); median time to progression was 9·4 months (95% CI 1·47-NE) in the nivolumab group and 19·53 months (2·33-NE) in the nivolumab plus ipilimumab group (HR 0·89, 95% CI 0·31-2·54). In an exploratory analysis, three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumour area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab.
Interpretation: Perioperative nivolumab alone and nivolumab plus ipilimumab appears to be safe and feasible in patients with resectable hepatocellular carcinoma. Our findings support further studies of immunotherapy in the perioperative setting in hepatocellular carcinoma.
Funding: Bristol Myers Squibb and the US National Institutes of Health.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests AOK reports consulting, advisory roles or stock ownership, or both, for Gilead Sciences, Bayer Health, Bristol-Myers Squibb, Exelixis, and Merck; reports research funding to the MD Anderson Cancer Center from Adaptimmune, Bayer/Onyx, Bristol Myers Squibb, Genentech, Hengrui Pharmaceutical, Hengrui Pharmaceutical, and Merck; honoraria from Bayer Health, Bristol Myers Squibb, Exelixis, and Merck; and other expenses from Bayer/Onyx, Bristol Myers Squibb, Exelixis, and Merck. EH reports research funding to the MD Anderson Cancer Center from Conquer Cancer Foundation, Kidney Cancer Association, and International Kidney Cancer Coalation. KPSR reports research funding to the MD Anderson Cancer Center from Bayer, Daiichi, AstraZeneca, Genentech, Guardant, and Merck; and consulting fees from Bayer, Daiichi, AstraZeneca; and honoraria from Bayer, and Daiichi. RS reports consulting fees from Boehringer Ingelheim. CWDT reports consulting fees donated to the MD Anderson Cancer Center from PanTher Therapeutics. RAW reports royalties or licenses from McGraw Hill for the MD Anderson Manual of Medical Oncology, 3rd edition; and travel support from Modern Medicine Emirates Oncology Society. JCY reports consulting fees from Hutchinson Medi Pharma, Ipsen Biopharmaceuticals, Amgen, Chiasma Pharma, Crinetics Pharmaceuticals, Advanced Accelerator Applications International, and Novartis Pharmaceuticals; and leadership roles at the North American Neuroendocrine Tumor Society. PS reports consulting fees from Achelois, Adaptive Biotechnologies, Affini-T, Apricity, BioAtla, BioNTech, Candel Therapeutics, Catalio, Codiak, Constellation, Dragonfly, Earli, Enable Medicine, Glympse, Hummingbird, ImaginAb, Infinity Pharma, Jounce, JSL Health, Lava Therapeutics, Lytix, Marker, Oncolytics, PBM Capital, Phenomic AI, Polaris Pharma, Sporos, Time Bioventures, Trained Therapeutix, Two Bear Capital, and Venn Biosciences; and ownership of stocks for Achelois, Adaptive Biotechnologies, Affini-T, Apricity, BioAtla, BioNTech, Candel Therapeutics, Catalio, Codiak, Constellation, Dragonfly, Earli, Enable Medicine, Glympse, Hummingbird, ImaginAb, Infinity Pharma, Jounce, JSL Health, Lava Therapeutics, Lytix, Marker, Oncolytics, PBM Capital, Phenomic AI, Polaris Pharma, Sporos, Time Bioventures, Trained Therapeutix, Two Bear Capital, and Venn Biosciences. JPA reports consulting fees from Achelois, Adaptive Biotechnologies, Apricity, BioAtla, BioNTech, Candel Therapeutics, Codiak, Dragonfly, Earli, Enable Medicine, Hummingbird, ImaginAb, Jounce, Lava Therapeutics, Lytix, Marker, PBM Capital, Phenomic AI, Polaris Pharma, Time Bioventures, Trained Therapeutix, Two Bear Capital, and Venn Biosciences; ownership of stocks for Achelois, Adaptive Biotechnologies, Apricity, BioAtla, BioNTech, Candel Therapeutics, Codiak, Dragonfly, Earli, Enable Medicine, Hummingbird, ImaginAb, Jounce, Lava Therapeutics, Lytix, Marker, PBM Capital, Phenomic AI, Polaris Pharma, Time Bioventures, Trained Therapeutix, Two Bear Capital, and Venn Biosciences. All other authors declare no competing interests.
Figures


Comment in
-
Exploring novel avenues for neoadjuvant treatment of hepatocellular carcinoma.Lancet Gastroenterol Hepatol. 2022 Mar;7(3):198-199. doi: 10.1016/S2468-1253(21)00462-3. Epub 2022 Jan 20. Lancet Gastroenterol Hepatol. 2022. PMID: 35065056 No abstract available.
References
-
- Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–55. - PubMed
-
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–54. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

