Reconstruction of cardiac position using body surface potentials
- PMID: 35065409
- PMCID: PMC8844250
- DOI: 10.1016/j.compbiomed.2021.105174
Reconstruction of cardiac position using body surface potentials
Abstract
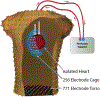
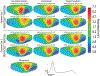
Electrocardiographic imaging (ECGI) is a noninvasive technique to assess the bioelectric activity of the heart which has been applied to aid in clinical diagnosis and management of cardiac dysfunction. ECGI is built on mathematical models that take into account several patient specific factors including the position of the heart within the torso. Errors in the localization of the heart within the torso, as might arise due to natural changes in heart position from respiration or changes in body position, contribute to errors in ECGI reconstructions of the cardiac activity, thereby reducing the clinical utility of ECGI. In this study we present a novel method for the reconstruction of cardiac geometry utilizing noninvasively acquired body surface potential measurements. Our geometric correction method simultaneously estimates the cardiac position over a series of heartbeats by leveraging an iterative approach which alternates between estimating the cardiac bioelectric source across all heartbeats and then estimating cardiac positions for each heartbeat. We demonstrate that our geometric correction method is able to reduce geometric error and improve ECGI accuracy in a wide range of testing scenarios. We examine the performance of our geometric correction method using different activation sequences, ranges of cardiac motion, and body surface electrode configurations. We find that after geometric correction resulting ECGI solution accuracy is improved and variability of the ECGI solutions between heartbeats is substantially reduced.
Keywords: Body surface potential mapping; Electrocardiographic imaging; Inverse problem; Optimization.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures




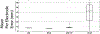



References
-
- Erkapic D, Neumann T, Ablation of premature ventricular complexes exclusively guided by three-dimensional noninvasive mapping., Card Electrophysiol Clin 7 (2015) 109–115. - PubMed
-
- Potyagaylo D, Segel M, Schulze WHW, Dössel O, Noninvasive Localization of Ectopic Foci: A New Optimization Approach for Simultaneous Reconstruction of Transmembrane Voltages and Epicardial Potentials BT, in: Ourselin S, Rueckert D, Smith N (Eds.), Functional Imaging and Modeling of the Heart, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 166–173.
-
- Cuculich P, Robinson C, Noninvasive ablation of ventricular tachycardia., N Engl J Med 378 (2018) 1651–1652. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

