The challenges of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing in low-middle income countries and possible cost-effective measures in resource-limited settings
- PMID: 35065670
- PMCID: PMC8783193
- DOI: 10.1186/s12992-022-00796-7
The challenges of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing in low-middle income countries and possible cost-effective measures in resource-limited settings
Abstract
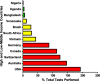
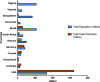
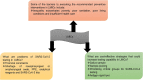
Diagnostic testing for the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection remains a challenge around the world, especially in low-middle-income countries (LMICs) with poor socio-economic backgrounds. From the beginning of the pandemic in December 2019 to August 2021, a total of approximately 3.4 billion tests were performed globally. The majority of these tests were restricted to high income countries. Reagents for diagnostic testing became a premium, LMICs either cannot afford or find manufacturers unwilling to supply them with expensive analytical reagents and equipment. From March to December 2020 obtaining testing kits for SARS-CoV-2 testing was a challenge. As the number of SARS-CoV-2 infection cases increases globally, large-scale testing still remains a challenge in LMICs. The aim of this review paper is to compare the total number and frequencies of SARS-CoV-2 testing in LMICs and high-income countries (HICs) using publicly available data from Worldometer COVID-19, as well as discussing possible interventions and cost-effective measures to increase testing capability in LMICs. In summary, HICs conducted more SARS-CoV-2 testing (USA: 192%, Australia: 146%, Switzerland: 124% and Canada: 113%) compared to middle-income countries (MICs) (Vietnam: 43%, South Africa: 29%, Brazil: 27% and Venezuela: 12%) and low-income countries (LICs) (Bangladesh: 6%, Uganda: 4% and Nigeria: 1%). Some of the cost-effective solutions to counteract the aforementioned problems includes using saliva instead of oropharyngeal or nasopharyngeal swabs, sample pooling, and testing high-priority groups to increase the number of mass testing in LMICs.
Keywords: Cost-effective strategies; Diagnostic testing challenges; Low-middle-income countries; Resource-limited settings; SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
There are no competing interests declared by the authors.
Figures
References
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;39(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Siu YL, Teoh KT, Lo J, Chan CM, Kien F, Escriou N, Tsao SW, Nicholls JM, Altmeyer R, Peiris JSM, Bruzzone R, Nal B. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J Virol. 2008;82(22):11318–11330. doi: 10.1128/JVI.01052-08. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

