Kinsenoside attenuates liver fibro-inflammation by suppressing dendritic cells via the PI3K-AKT-FoxO1 pathway
- PMID: 35066108
- PMCID: PMC8776354
- DOI: 10.1016/j.phrs.2022.106092
Kinsenoside attenuates liver fibro-inflammation by suppressing dendritic cells via the PI3K-AKT-FoxO1 pathway
Abstract
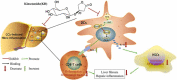
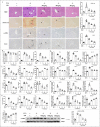
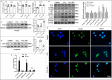
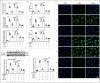
Kinsenoside (KD) exhibits anti-inflammatory and immunosuppressive effects. Dendritic cells (DCs) are critical regulators of the pathologic inflammatory milieu in liver fibrosis (LF). Herein, we explored whether and how KD repressed development of LF via DC regulation and verified the pathway involved in the process. Given our analysis, both KD and adoptive transfer of KD-conditioned DCs conspicuously reduced hepatic histopathological damage, proinflammatory cytokine release and extracellular matrix deposition in CCl4-induced LF mice. Of note, KD restrained the LF-driven rise in CD86, MHC-II, and CCR7 levels and, simultaneously, upregulated PD-L1 expression on DCs specifically, which blocked CD8+T cell activation. Additionally, KD reduced DC glycolysis, maintained DCs immature, accompanied by IL-12 decrease in DCs. Inhibiting DC function by KD disturbed the communication of DCs and HSCs with the expression or secretion of α-SMA and Col-I declined in the liver. Mechanistically, KD suppressed the phosphorylation of PI3K-AKT driven by LF or PI3K agonist, followed by enhanced nuclear transport of FoxO1 and upregulated interaction of FoxO1 with the PD-L1 promoter in DCs. PI3K inhibitor or si-IL-12 acting on DC could relieve LF, HSC activation and diminish the effect of KD. In conclusion, KD suppressed DC maturation with promoted PD-L1 expression via PI3K-AKT-FoxO1 and decreased IL-12 secretion, which blocked activation of CD8+T cells and HSCs, thereby alleviating liver injury and fibro-inflammation in LF.
Keywords: 740 Y-P (PubChem CID:90488730); Dendritic cell; Interleukin-12; Kinsenoside; Kinsenoside (PubChem CID:10422896); LY294002 (PubChem CID:3973); Liver fibro-inflammation; PI3K-AKT-FoxO1 axis; Programmed cell death ligand 1.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Connolly M.K., Bedrosian A.S., Mallen-St Clair J., Mitchell A.P., Ibrahim J., Stroud A., Pachter H.L., Bar-Sagi D., Frey A.B., Miller G. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J. Clin. Invest. 2009;119:3213–3225. doi: 10.1172/jci37581. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

