Esmolol to Treat the Hemodynamic Effects of Septic Shock: A Randomized Controlled Trial
- PMID: 35066509
- PMCID: PMC10448435
- DOI: 10.1097/SHK.0000000000001905
Esmolol to Treat the Hemodynamic Effects of Septic Shock: A Randomized Controlled Trial
Abstract
Introduction: Septic shock is often characterized by tachycardia and a hyperdynamic hemodynamic profile. Use of the beta antagonist esmolol has been proposed as a therapy to lower heart rate, thereby improving diastolic filling time and improving cardiac output, resulting in a reduction in vasopressor support.
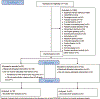
Methods: We conducted a two-center, open-label, randomized, Phase II trial comparing esmolol to placebo in septic shock patients with tachycardia. The primary endpoint was improvement in hemodynamics as measured by the difference in norepinephrine equivalent dose (NED) between groups at 6 hours after initiation of study drug. Secondary outcomes included assessing differences in inflammatory biomarkers and oxygen consumption (VO2).
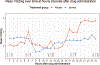
Results: A total of 1,122 patients were assessed for eligibility and met inclusion criteria; 42 underwent randomization, and 40 received study interventions (18 in the esmolol arm and 22 in the usual care arm). The mean NED at 6 h was 0.30 ± 0.17 mcg/kg/min in the esmolol arm compared to 0.21 ± 0.19 in the standard care arm (P = 0.15). There was no difference in number of shock free days between the esmolol (2, IQR 0, 5) and control groups (2.5, IQR 0, 6) (P = 0.32). There were lower levels of C-reactive protein at 12 and 24 h in the esmolol arm, as well as a statistically significant difference in trend over time between groups. There were no differences in terms of IL-4, IL-6, IL-10, and TNFα. Among a subset who underwent VO2 monitoring, there was decreased oxygen consumption in the esmolol patients; the mean difference between groups at 24 h was -2.07 mL/kg/min (95% CI -3.82, -0.31) (P = 0.02), with a significant difference for the trend over time (P < 0.01).
Conclusion: Among patients with septic shock, infusion of esmolol did not improve vasopressor requirements or time to shock reversal. Esmolol was associated with decreased levels of C-reactive protein over 24 h.
Trial registration: www.clinicaltrials.gov. Registered February 24, 2015, https://clinicaltrials.gov/ct2/show/NCT02369900.
Copyright © 2022 by the Shock Society.
Conflict of interest statement
The authors report no conflict of interests.
Figures


References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR: Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29(7):1303–1310, 2001. - PubMed
-
- Rudiger A, Singer M: Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35(6):1599–1608, 2007. - PubMed
-
- Schmittinger CA, Dunser MW, Torgersen C, Luckner G, Lorenz I, Schmid S, Joannidis M, Moser P, Hasibeder WR, Halabi M, et al.: Histologic pathologies of the myocardium in septic shock: a prospective observational study. Shock 39(4):329–335, 2013. - PubMed
-
- Dunser MW, Hasibeder WR: Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med 24(5): 293–316, 2009. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

