T cell apoptosis characterizes severe Covid-19 disease
- PMID: 35066575
- PMCID: PMC8782710
- DOI: 10.1038/s41418-022-00936-x
T cell apoptosis characterizes severe Covid-19 disease
Abstract
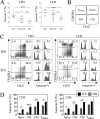
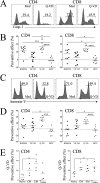
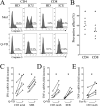
Severe SARS-CoV-2 infections are characterized by lymphopenia, but the mechanisms involved are still elusive. Based on our knowledge of HIV pathophysiology, we hypothesized that SARS-CoV-2 infection-mediated lymphopenia could also be related to T cell apoptosis. By comparing intensive care unit (ICU) and non-ICU COVID-19 patients with age-matched healthy donors, we found a strong positive correlation between plasma levels of soluble FasL (sFasL) and T cell surface expression of Fas/CD95 with the propensity of T cells to die and CD4 T cell counts. Plasma levels of sFasL and T cell death are correlated with CXCL10 which is part of the signature of 4 biomarkers of disease severity (ROC, 0.98). We also found that members of the Bcl-2 family had modulated in the T cells of COVID-19 patients. More importantly, we demonstrated that the pan-caspase inhibitor, Q-VD, prevents T cell death by apoptosis and enhances Th1 transcripts. Altogether, our results are compatible with a model in which T-cell apoptosis accounts for T lymphopenia in individuals with severe COVID-19. Therefore, a strategy aimed at blocking caspase activation could be beneficial for preventing immunodeficiency in COVID-19 patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Worobey M. Dissecting the early COVID-19 cases in Wuhan. Science. 2021;374:1202–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

