Association of Circulating Proteins with Death or Lung Transplant in Patients with Idiopathic Pulmonary Fibrosis in the IPF-PRO Registry Cohort
- PMID: 35066606
- PMCID: PMC8881240
- DOI: 10.1007/s00408-021-00505-y
Association of Circulating Proteins with Death or Lung Transplant in Patients with Idiopathic Pulmonary Fibrosis in the IPF-PRO Registry Cohort
Erratum in
-
Correction to: Association of Circulating Proteins with Death or Lung Transplant in Patients with Idiopathic Pulmonary Fibrosis in the IPF-PRO Registry Cohort.Lung. 2022 Feb;200(1):19. doi: 10.1007/s00408-022-00516-3. Lung. 2022. PMID: 35166906 Free PMC article. No abstract available.
Abstract
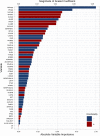
Idiopathic pulmonary fibrosis (IPF) is a progressive and ultimately fatal disease with a variable clinical course. Biomarkers that predict patient outcomes are needed. We leveraged data from 300 patients in the multicenter IPF-PRO Registry to determine associations between circulating proteins and the composite outcome of respiratory death or lung transplant. Plasma collected at enrollment was analyzed using aptamer-based proteomics (1305 proteins). Over a median follow-up of 30.4 months, there were 76 respiratory deaths and 26 lung transplants. In unadjusted univariable analyses, 61 proteins were significantly associated with the outcome (hazard ratio > 2 or < 0.5, corrected p ≤ 0.05). In multivariable analyses, a set of 4 clinical measures and 47 unique proteins predicted the probability of respiratory death or lung transplant with an optimism-corrected C-index of 0.76. Our results suggest that select circulating proteins strongly associate with the risk of mortality in patients with IPF and confer information independent of clinical measures.
Keywords: Biomarkers; Interstitial lung diseases; Observational study; Proteomics.
© 2022. The Author(s).
Conflict of interest statement
The authors have reported to Lung the following conflicts of interest: JLT, MLN, RO, HM and SMP are employees of the Duke Clinical Research Institute, which was funded by Boehringer Ingelheim Pharmaceuticals, Inc to conduct this research. JR reports grants and personal fees from Boehringer Ingelheim and grants from Genentech, Galapagos, Syneos Health, Bellerophon Therapeutics, FibroGen, and the National Institutes of Health. JAL reports personal fees from Biogen, Boehringer Ingelheim, Galecto, Roche/Genentech and Veracyte. JAdA reports personal fees from Boehringer Ingelheim. MG reports personal fees, non-financial support, and other support from the France Foundation; grants, non-financial support and other support from Boehringer Ingelheim and the Pulmonary Fibrosis Foundation; and personal fees from Genentech. HH is on a speaker panel for Boehringer Ingelheim. IN reports personal fees from Boehringer Ingelheim, Genentech, and ImmuneWorks. JAB has no disclosures. KRF reports grants and personal fees from Boehringer Ingelheim and Roche/Genentech and personal fees from FibroGen, Sanofi, Genzyme, and Veracyte. TBL was an employee of Boehringer Ingelheim at the time this study was conducted. CH is an employee of Boehringer Ingelheim.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

