Glial Cells Response in Stroke
- PMID: 35066715
- PMCID: PMC11415215
- DOI: 10.1007/s10571-021-01183-3
Glial Cells Response in Stroke
Abstract
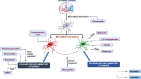
As the second-leading cause of death, stroke faces several challenges in terms of treatment because of the limited therapeutic interventions available. Previous studies primarily focused on metabolic and blood flow properties as a target for treating stroke, including recombinant tissue plasminogen activator and mechanical thrombectomy, which are the only USFDA approved therapies. These interventions have the limitation of a narrow therapeutic time window, the possibility of hemorrhagic complications, and the expertise required for performing these interventions. Thus, it is important to identify the contributing factors that exacerbate the ischemic outcome and to develop therapies targeting them for regulating cellular homeostasis, mainly neuronal survival and regeneration. Glial cells, primarily microglia, astrocytes, and oligodendrocytes, have been shown to have a crucial role in the prognosis of ischemic brain injury, contributing to inflammatory responses. They play a dual role in both the onset as well as resolution of the inflammatory responses. Understanding the different mechanisms driving these effects can aid in the development of therapeutic targets and further mitigate the damage caused. In this review, we summarize the functions of various glial cells and their contribution to stroke pathology. The review highlights the therapeutic options currently being explored and developed that primarily target glial cells and can be used as neuroprotective agents for the treatment of ischemic stroke.
Keywords: Blood–brain barrier; Glial cells; Ischemic stroke; Neuroprotection; Therapeutic target.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard MJS (2011) The Hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science 334(6063):1727–1731 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

