Maternal Nanomaterial Inhalation Exposure: Critical Gestational Period in the Uterine Microcirculation is Angiotensin II Dependent
- PMID: 35066857
- PMCID: PMC9013006
- DOI: 10.1007/s12012-021-09712-8
Maternal Nanomaterial Inhalation Exposure: Critical Gestational Period in the Uterine Microcirculation is Angiotensin II Dependent
Abstract
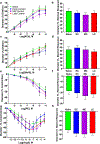
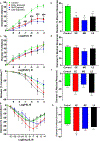
Maternal inhalation exposure to engineered nanomaterials (ENM) has been associated with microvascular dysfunction and adverse cardiovascular responses. Pregnancy requires coordinated vascular adaptation and growth that are imperative for survival. Key events in pregnancy hallmark distinct periods of gestation such as implantation, spiral artery remodeling, placentation, and trophoblast invasion. Angiotensin II (Ang II) is a critical vasoactive mediator responsible for adaptations and is implicated in the pathology of preeclampsia. If perturbations occur during gestation, such as those caused by ENM inhalation exposure, then maternal-fetal health consequences may occur. Our study aimed to identify the period of gestation in which maternal microvascular functional and fetal health are most vulnerable. Additionally, we wanted to determine if Ang II sensitivity and receptor density is altered due to exposure. Dams were exposed to ENM aerosols (nano-titanium dioxide) during three gestational windows: early (EE, gestational day (GD) 2-6), mid (ME, GD 8-12) or late (LE, GD 15-19). Within the EE group dry pup mass decreased by 16.3% and uterine radial artery wall to lumen ratio (WLR) increased by 25.9%. Uterine radial artery response to Ang II sensitivity increased by 40.5% in the EE group. Ang II receptor density was altered in the EE and LE group with decreased levels of AT2R. We conclude that early gestational maternal inhalation exposures resulted in altered vascular anatomy and physiology. Exposure during this time-period results in altered vascular reactivity and changes to uterine radial artery WLR, leading to decreased perfusion to the fetus and resulting in lower pup mass.
Keywords: Angiotensin II; Gestational inhalation exposure; Microcirculation; Titanium dioxide; Toxicology.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures

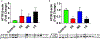
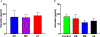
References
-
- Abukabda AB, McBride CR, Batchelor TP, Goldsmith WT, Bowdridge EC, Garner KL, Friend S, & Nurkiewicz TR (2018). Group II innate lymphoid cells and microvascular dysfunction from pulmonary titanium dioxide nanoparticle exposure. Particle and Fibre Toxicology, 15(1), 43. 10.1186/s12989-018-0280-2 - DOI - PMC - PubMed
-
- Rossi S, Savi M, Mazzola M, Pinelli S, Alinovi R, Gennaccaro L, Pagliaro A, Meraviglia V, Galetti M, Lozano-Garcia O, Rossini A, Frati C, Falco A, Quaini F, Bocchi L, Stilli D, Lucas S, Goldoni M, Macchi E, … Miragoli M (2019). Subchronic exposure to titanium dioxide nanoparticles modifies cardiac structure and performance in spontaneously hypertensive rats. Particle and Fibre Toxicology, 16(1), 25. 10.1186/s12989-019-0311-7 - DOI - PMC - PubMed
-
- Abukabda AB, Bowdridge EC, McBride CR, Batchelor TP, Goldsmith WT, Garner KL, Friend S, & Nurkiewicz TR (2019). Maternal titanium dioxide nanomaterial inhalation exposure compromises placental hemodynamics. Toxicology and Applied Pharmacology, 367, 51–61. 10.1016/j.taap.2019.01.024 - DOI - PMC - PubMed
-
- Bowdridge EC, Abukabda AB, Engles KJ, McBride CR, Batchelor TP, Goldsmith WT, Garner KL, Friend S, & Nurkiewicz TR (2019). Maternal engineered nanomaterial inhalation during gestation disrupts vascular kisspeptin reactivity. Toxicological Sciences, 169(2), 524–533. 10.1093/toxsci/kfz064 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

