AAPM Task Group Report 290: Respiratory motion management for particle therapy
- PMID: 35066871
- PMCID: PMC9306777
- DOI: 10.1002/mp.15470
AAPM Task Group Report 290: Respiratory motion management for particle therapy
Abstract
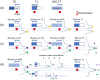
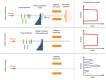
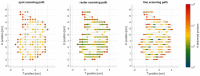
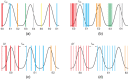
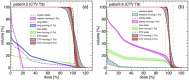
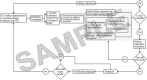
Dose uncertainty induced by respiratory motion remains a major concern for treating thoracic and abdominal lesions using particle beams. This Task Group report reviews the impact of tumor motion and dosimetric considerations in particle radiotherapy, current motion-management techniques, and limitations for different particle-beam delivery modes (i.e., passive scattering, uniform scanning, and pencil-beam scanning). Furthermore, the report provides guidance and risk analysis for quality assurance of the motion-management procedures to ensure consistency and accuracy, and discusses future development and emerging motion-management strategies. This report supplements previously published AAPM report TG76, and considers aspects of motion management that are crucial to the accurate and safe delivery of particle-beam therapy. To that end, this report produces general recommendations for commissioning and facility-specific dosimetric characterization, motion assessment, treatment planning, active and passive motion-management techniques, image guidance and related decision-making, monitoring throughout therapy, and recommendations for vendors. Key among these recommendations are that: (1) facilities should perform thorough planning studies (using retrospective data) and develop standard operating procedures that address all aspects of therapy for any treatment site involving respiratory motion; (2) a risk-based methodology should be adopted for quality management and ongoing process improvement.
Keywords: motion management; particle therapy.
© 2022 The Authors. Medical Physics published by Wiley Periodicals LLC on behalf of American Association of Physicists in Medicine.
Conflict of interest statement
The members of TG‐290 listed below attest that they have no potential Conflict of Interest related to the subject matter or materials presented in this document: Heng Li, Lei Dong, Christoph Bert, Joe Chang, Stella Flampouri, Kyung‐Wook Jee, Liyong Lin, Michael Moyers, Shinichiro Mori, Joerg Rottmann, Erik Tryggestad, Sastry Vedam.
Figures





References
-
- Zhang X, Li Y, Pan X, et al. Intensity‐modulated proton therapy reduces the dose to normal tissue compared with intensity‐modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non‐small‐cell lung cancer: a virtual clinical study. Int J Radiat Oncol Biol Phys. 2010; 77(2): 357–366. - PMC - PubMed
-
- Wang X, Krishnan S, Zhang X, et al. Proton radiotherapy for liver tumors: dosimetric advantages over photon plans. Med Dosim. 2008; 33(4): 259–267. - PubMed
-
- Doyen J, Falk AT, Floquet V, Hérault J, Hannoun‐Lévi J‐M. Proton beams in cancer treatments: clinical outcomes and dosimetric comparisons with photon therapy. Cancer Treat Rev. 2016; 43: 104–112. - PubMed
-
- Engelsman M, Schwarz M, Dong L. Physics controversies in proton therapy. Semin Radiat Oncol. 2013;23(2):88–96. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

