Percutaneous decannulation of extracorporeal membrane oxygenation using a plug-based closure device
- PMID: 35067004
- PMCID: PMC9541842
- DOI: 10.1002/ccd.30096
Percutaneous decannulation of extracorporeal membrane oxygenation using a plug-based closure device
Abstract
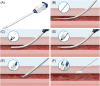
Background: There is limited experience of using the MANTA plug-based vascular closure device for percutaneous arterial closure of the femoral artery after venoarterial extracorporeal membrane oxygenation.
Objectives: To study femoral artery complications and need for subsequent vascular interventions after percutaneous decannulation of venoarterial extracorporeal membrane oxygenation (VA ECMO) using the MANTA plug-based vascular closure device.
Methods: We studied 34 consecutive patients who underwent percutaneous decannulation of VA ECMO using the MANTA device. Primary outcomes were conversion to surgical cutdown of the groin at decannulation (immediate) or later. Secondary outcomes were type of vascular complication necessitating conversion to surgical cutdown of the groin.
Results: Six (17.7%) patients had to undergo immediate (n = 3) or late (n = 3) conversion to surgical cutdown of the groin. Of these, three were owing to occlusion of the common femoral artery resulting in insufficient distal perfusion and three owing to bleeding or pseudoaneurysm. The mechanism of failure was complete intravascular deployment of the MANTA device in three patients, incomplete MANTA sealing of the arteriotomy in one patient, MANTA-unrelated thrombotic occlusion in one patient, and unknown in one patient. Surgical cut-down was typically performed with concomitant catheter thrombectomy with or without patch reconstruction of the artery.
Conclusion: Percutaneous decannulation of VA ECMO using the MANTA VCD was feasible but a substantial number of patients needed to be converted to unplanned surgical repair, owing to either closure site-located stenosis/occlusion or bleeding. If suboptimal MANTA positioning is suspected, a low threshold for conversion to surgical cutdown of the groin is recommended.
Keywords: MANTA; vascular closure device; venoarterial extracorporeal membrane oxygenation.
© 2022 The Authors. Catheterization and Cardiovascular Interventions published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
References
-
- Guglin M, Zucker MJ, Bazan VM, et al. Venoarterial ECMO for adults: JACC scientific expert panel. J Am Coll Cardiol. 2019;73:698‐716. - PubMed
-
- Salna M, Takayama H, Garan AR, et al. Incidence and risk factors of groin lymphocele formation after venoarterial extracorporeal membrane oxygenation in cardiogenic shock patients. J Vasc Surg. 2018;67:542‐548. - PubMed
-
- Kastengren M, Svenarud P, Källner G, Settergren M, Franco‐Cereceda A, Dalén M. Percutaneous vascular closure device in minimally invasive mitral valve surgery. Ann Thorac Surg. 2020;110:85‐91. - PubMed
-
- Hwang JW, Yang JH, Sung K, et al. Percutaneous removal using Perclose ProGlide closure devices versus surgical removal for weaning after percutaneous cannulation for venoarterial extracorporeal membrane oxygenation. J Vasc Surg. 2016;63:998‐1003. - PubMed
-
- Wood DA, Krajcer Z, Sathananthan J, et al. Pivotal clinical study to evaluate the safety and effectiveness of the MANTA percutaneous vascular closure device. Circ Cardiovasc Interv. 2019;12:e007258. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

