RNA-based therapeutics for neurological diseases
- PMID: 35067193
- PMCID: PMC8786337
- DOI: 10.1080/15476286.2021.2021650
RNA-based therapeutics for neurological diseases
Abstract
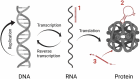
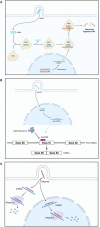
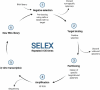
RNA-based therapeutics have entered the mainstream with seemingly limitless possibilities to treat all categories of neurological disease. Here, common RNA-based drug modalities such as antisense oligonucleotides, small interfering RNAs, RNA aptamers, RNA-based vaccines and mRNA drugs are reviewed highlighting their current and potential applications. Rapid progress has been made across rare genetic diseases and neurodegenerative disorders, but safe and effective delivery to the brain remains a significant challenge for many applications. The advent of individualized RNA-based therapies for ultra-rare diseases is discussed against the backdrop of the emergence of this field into more common conditions such as Alzheimer's disease and ischaemic stroke. There remains significant untapped potential in the use of RNA-based therapeutics for behavioural disorders and tumours of the central nervous system; coupled with the accelerated development expected over the next decade, the true potential of RNA-based therapeutics to transform the therapeutic landscape in neurology remains to be uncovered.
Keywords: RNA; RNA aptamer; RNA therapeutics; RNA vaccine; antisense oligonucleotide; exon skipping; neurological disease; siRNA.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
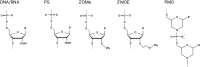

References
-
- Anthony K, Gallo J-M.. Aberrant RNA processing events in neurological disorders. Brain Res. 2010;1338:67–77. - PubMed
-
- Mansfield SG, Chao H, Walsh CE. RNA repair using spliceosome-mediated RNA trans-splicing. Trends Mol Med. 2004;10:263–268. - PubMed
-
- Aartsma-Rus A. Overview on AON design. Methods Mol Biol. 2012;867:117–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
