Structural maturation of the HIV-1 RNA 5' untranslated region by Pr55Gag and its maturation products
- PMID: 35067194
- PMCID: PMC8786341
- DOI: 10.1080/15476286.2021.2021677
Structural maturation of the HIV-1 RNA 5' untranslated region by Pr55Gag and its maturation products
Abstract
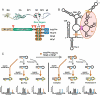
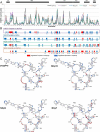
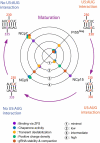
Maturation of the HIV-1 viral particles shortly after budding is required for infectivity. During this process, the Pr55Gag precursor undergoes a cascade of proteolytic cleavages, and whilst the structural rearrangements of the viral proteins are well understood, the concomitant maturation of the genomic RNA (gRNA) structure is unexplored, despite evidence that it is required for infectivity. To get insight into this process, we systematically analysed the interactions between Pr55Gag or its maturation products (NCp15, NCp9 and NCp7) and the 5' gRNA region and their structural consequences, in vitro. We show that Pr55Gag and its maturation products mostly bind at different RNA sites and with different contributions of their two zinc knuckle domains. Importantly, these proteins have different transient and permanent effects on the RNA structure, the late NCp9 and NCp7 inducing dramatic structural rearrangements. Altogether, our results reveal the distinct contributions of the different Pr55Gag maturation products on the gRNA structural maturation.
Keywords: Gag; HIV-1; NCp15; NCp7; NCp9; Pr55Gag; RNA chaperone; RNA structure; genomic RNA; maturation.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Swanstrom R, Wills JW.. Synthesis, assembly, and processing of viral proteins [Internet]. In: Coffin JM, Hughes SH, and Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY):Cold Spring Harbor Laboratory Press; 1997. Available from: http://www.ncbi.nlm.nih.gov/books/NBK19456/ - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
