Kinetics of SARS-CoV-2-Neutralising Antibodies of Residents of Long-Term Care Facilities
- PMID: 35067704
- PMCID: PMC8683825
- DOI: 10.1007/s12603-021-1713-4
Kinetics of SARS-CoV-2-Neutralising Antibodies of Residents of Long-Term Care Facilities
Abstract
Introduction: Elderly residents of nursing homes (NHs) and long-term care units (LTCUs) have been shown to have a high risk of mortality and morbidity in cases of SARS-CoV-2 infection. The objective of this study was to examine the kinetics of neutralizing antibodies (NAbs) directed against the SARS-CoV-2 virus in residents of the NH and LTCU units of our University Hospital who were identified with positive serology after the first epidemic outbreak.
Materials and methods: The participants included were sampled every three months for qualitative serological testing, as well as quantitative testing by neutralization tests using retroviral particles containing the S glycoprotein of SARS-CoV-2. Vaccination using the Comirnaty (Pfizer BNT162b2) vaccine begun before the last serological follow-up.
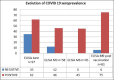
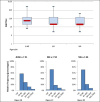
Results: The median NAb titer in June 2020 was 80 [40; 60] versus 40 [40; 160] three months later, showing a statistically significant decline (p < 0.007), but remained stable between the three- and six-month timepoints (p = 0.867). By nine months after vaccination, we observed a significant difference between vaccinated residents known to have positive serology before vaccination (SERO+, Vacc+) and those vaccinated without having previously shown COVID-19 seroconversion (SERO-, Vacc+), the latter group showing similar titers to the SERO+, Vacc- participants (p=0.166). The median antibody titer in SERO+, Vacc+ patients increased 15-fold following vaccination.
Discussion: Humoral immunity against SARS-CoV-2 appears to be persistent in elderly institutionalized patients, with a good post-vaccination response by residents who had already shown seroconversion but a notably diminished response by those who were seronegative before vaccination. To evaluate immunity in its entirety and elaborate a sound vaccination strategy, the cellular immune response via T cells specific to SARS-CoV-2 merits analysis, as this response is susceptible to being affected by immunosenescence.
Keywords: COVID-19; SARS-CoV-2; immunosenescence; nursing homes; serological assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mo P, Xing Y, Xiao Y, et al. (2020) Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. doi: 10.1093/cid/ciaa270. - DOI
-
- Morley JE, Vellas B. COVID-19 and Older Adult. J Nutr Health Aging. 2020;24:364–365. 10.1007/s12603-020-1349-9 - DOI - PMC - PubMed
-
- Etard J-F, Vanhems P, Atlani-Duault L, Ecochard R. Potential lethal outbreak of coronavirus disease (COVID-19) among the elderly in retirement homes and long-term facilities, France, March 2020. Euro Surveill. 2020;25(15) 10.2807/1560-7917.ES.2020.25.15.2000448 - DOI - PMC - PubMed
-
- O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590(7844):140–145. 10.1038/s41586-020-2918-0 - DOI - PubMed
-
- Plaçais L, Richier Q [ COVID-19: Clinical, biological and radiological characteristics in adults, infants and pregnant women. An up-to-date review at the heart of the pandemic. Rev Med Interne. 2020;41(5):308–318. 10.1016/j.revmed.2020.04.004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

