SARS-CoV-2 infection in dialysis and kidney transplant patients: immunological and serological response
- PMID: 35067905
- PMCID: PMC8784230
- DOI: 10.1007/s40620-021-01214-8
SARS-CoV-2 infection in dialysis and kidney transplant patients: immunological and serological response
Abstract
Background: Dialysis and kidney transplant patients with moderate-severe COVID-19 have a high mortality rate, around 30%, that is similar in the two populations, despite differences in their baseline characteristics. In these groups, the immunology of the disease has been poorly explored.
Methods: Thirty-two patients on dialysis or with kidney transplant and SARS-CoV-2 infection requiring hospitalization (COV group) were included in our study. Lymphocyte subsets, dendritic cell (DC) counts and monocyte activation were studied. SARS-CoV-2 anti-spike/anti-nucleocapsid were monitored, and baseline cytokines and chemokines were measured in 10 patients.
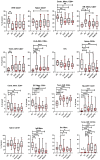
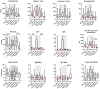
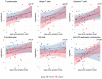
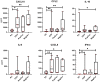
Results: The COV group, compared to healthy subjects and uninfected dialysis/kidney transplant controls, showed lower numbers of CD4 + and CD8 + T cells, Natural-Killer (NK), B cells, plasmacytoid and myeloid DCs, while the proportion of terminally differentiated B-cells was increased. IL6, IL10, IFN-α and chemokines involved in monocyte and neutrophil recruitment were higher in the COV group, compared to uninfected dialysis/kidney transplant controls. Patients with severe disease had lower CD4 + , CD8 + and B-cell counts and lower monocyte HLA-DR expression. Of note, when comparing dialysis and kidney transplant patients with COVID-19, the latter group presented lower NK and pDC counts and monocyte HLA-DR expression. Up to 60 days after symptom onset, kidney transplant recipients showed lower levels of anti-spike antibodies compared to dialysis patients.
Conclusions: During SARS-CoV-2 infection, dialysis and kidney transplant patients manifest immunophenotype abnormalities; these are similar in the two groups, however kidney transplant recipients show more profound alterations of the innate immune system and lower anti-spike antibody response.
Keywords: COVID-19; Hemodialysis; Kidney transplant; Lymphocytes; SARS-CoV-2.
© 2021. The Author(s) under exclusive licence to Italian Society of Nephrology.
Conflict of interest statement
FA, consultancy fees from Baxter, AstraZeneca and Otsuka, advisory board for Trevere Therapeutics; AMR research funding from AstraZeneca, European Hematology Association, Transcan2-ERANET, Italian Association for Cancer Research (Fondazione AIRC); advisory board for from Amgen, Celgene, Takeda, Janssen.
Figures

References
-
- Bergamaschi L, Mescia F, Turner L, Hanson AL, Kotagiri P, Dunmore BJ, et al. (2021) Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity [Internet]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1074761321002168. Accessed 2021 Jun 5 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

