Immune dynamics in the CNS and its barriers during homeostasis and disease
- PMID: 35067941
- PMCID: PMC8852772
- DOI: 10.1111/imr.13066
Immune dynamics in the CNS and its barriers during homeostasis and disease
Abstract
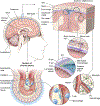
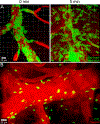
The central nervous system (CNS) has historically been viewed as an immunologically privileged site, but recent studies have uncovered a vast landscape of immune cells that reside primarily along its borders. While microglia are largely responsible for surveying the parenchyma, CNS barrier sites are inhabited by a plethora of different innate and adaptive immune cells that participate in everything from the defense against microbes to the maintenance of neural function. Static and dynamic imaging studies have revolutionized the field of neuroimmunology by providing detailed maps of CNS immune cells as well as information about how these cells move, organize, and interact during steady-state and inflammatory conditions. These studies have also redefined our understanding of neural-immune interactions at a cellular level and reshaped our conceptual view of immune privilege in this specialized compartment. This review will focus on insights gained using imaging techniques in the field of neuroimmunology, with an emphasis on anatomy and CNS immune dynamics during homeostasis, infectious diseases, injuries, and aging.
Keywords: Alzheimer's; brain; dura; glymphatics; infection; intravital; lymphatics; meninges; microglia; monocytes; neuroimmunology; neutrophils; sinuses; stroke; traumatic brain injury; two-photon microscopy; vasculature; virus.
Published 2022. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Conflicts of Interests
The authors have no conflicts of interest to report.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

