Patient and Caregiver Preferences for First-Line Treatments of Metastatic Non-Small Cell Lung Cancer: A Discrete Choice Experiment
- PMID: 35068928
- PMCID: PMC8769053
- DOI: 10.2147/PPA.S338840
Patient and Caregiver Preferences for First-Line Treatments of Metastatic Non-Small Cell Lung Cancer: A Discrete Choice Experiment
Abstract
Purpose: The approval of immune checkpoint inhibitors for metastatic non-small-cell lung carcinomas (mNSCLC) treatment has presented more care options. Therefore, it is important to identify the benefit-risk trade-offs patients and caregivers are willing to make among potential treatment options. This study quantified the preferences of patients and caregivers for attributes of mNSCLC treatment.
Methods: Patients with mNSCLC and caregivers completed an online survey assessing preferences using a discrete choice experiment. Respondents chose between hypothetical treatment profiles, with varying levels for 7 attributes associated with first-line treatment, including overall survival (OS), progression-free survival, select adverse events (AEs), and regimen (caregivers). Hierarchical Bayesian modeling was used to estimate attribute-level preference weights.
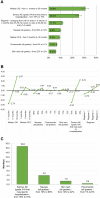
Results: Patients (n = 308) and caregivers (n = 166) most valued increasing OS from 11 to 30 months, followed by decreasing the risk of a serious AE (grade 3/4) that may lead to hospitalization from 70% to 18%. These attributes were over twice as important to both sets of respondents as the other attributes measured. Patients and caregivers would accept increases in the risks of a serious AE (grade 3/4) from 18% to 70% and all grades nausea from 10% to 69% if OS increased by 16.8 and 4.0 months, respectively. The least valued attributes were all grades of pneumonitis (patients) and all grades of skin rash (caregivers).
Conclusion: Patients and caregivers are willing to make trade-offs between efficacy and toxicity and may require up to 1.5 years of increased OS to accept a higher risk of AEs. These results can provide guidance to oncologists when engaging in shared-decision making discussions.
Keywords: immune checkpoint inhibitors; metastases; non-small-cell lung carcinomas; overall survival; patient preference; toxicities.
© 2022 Yong et al.
Conflict of interest statement
Candice Yong and Brian Seal were employees and shareholders of AstraZeneca at the time of study conduct. Ion Cotarla is an employee and shareholder of AstraZeneca. M. Janelle Cambron-Mellott, Oliver Will, Martine C. Maculaitis, Kelly Clapp, and Emily Mulvihill are employees of Cerner Enviza, which received funding from AstraZeneca for conducting the study and analysis and for manuscript preparation. Ranee Mehra is an employee of University of Maryland Marlene and Stewart Greenebaum Cancer Center, which received research funding from AstraZeneca for consulting on the study. Ranee Mehra’s institution has also received research funding outside of this work from AstraZeneca and Merck, and she has received consulting fees from Rakuten Medical, outside of this work. The authors report no other conflicts of interest in this work.
Figures

References
-
- Surveillance Epidemiology and End Results (SEER) Program. Cancer Stat Facts: Common Cancer Sites. National Cancer Institute; 2020.
-
- American Cancer Society. What is Lung Cancer?; 2019.
-
- Kelly K, Crowley J, Bunn PA Jr, et al. Randomized Phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19(13):3210–3218. doi:10.1200/JCO.2001.19.13.3210 - DOI - PubMed
-
- Fossella FV, DeVore R, Kerr RN, et al. Randomized Phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18(12):2354–2362. - PubMed
LinkOut - more resources
Full Text Sources

