Patients and physician satisfaction of Degarelix in androgen deprivation therapy for advanced hormone-dependent prostate cancer in the Netherlands
- PMID: 35069083
- PMCID: PMC8772641
- DOI: 10.1097/CU9.0000000000000029
Patients and physician satisfaction of Degarelix in androgen deprivation therapy for advanced hormone-dependent prostate cancer in the Netherlands
Abstract
Background: To explore the effectiveness and safety of the gonadotropin-releasing hormone antagonist, Degarelix, for the treatment of advanced hormone-dependent prostate cancer (PCa) in a real-world setting.
Methods: In this noninterventional study, patients with advanced hormone-dependent PCa were included. Primary endpoints were progression-free survival (PFS) failure defined as either prostate-specific antigen failure, additional therapy related to PCa, or death. Secondary endpoints included patient and physician satisfaction scores, urinary symptoms, and adverse events (AEs).
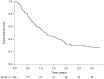
Results: Of 274 patients with PCa, 271 received at least 1 dose of Degarelix. At a median follow-up of 12.2 (interquartile range 6.2-22.0) months, 148 patients (60.2%) had PFS failure. Thirty-five patients (13%) withdrew from the study due to AEs, 23 patients (8.4%) died, and 36 patients (13%) completed 3 years' follow-up. Urinary symptoms significantly decreased over time. In the safety population, 87.8% of patients reported AEs, with injection-site reactions commonly reported. The majority of physicians and patients considered the therapy satisfactory and well tolerated.
Conclusions: In this observational study, Degarelix treatment was well accepted by men with advanced hormone-dependent PCa. Compared with phase III studies, a higher proportion of patients had PFS failure, possibly due to the inclusion of men with more advanced disease in the current study, and more men reported AEs.
Keywords: Androgen antagonists; Clinical trials; Degarelix; Prostate-specific antigen; Prostatic neoplasms.
Copyright © 2021 The Authors. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
H Roshani: no conflict of interest. EPM Roos, HW Elzevier, C van de Beek, and RCM Pelger: received study fees from Ferring. P van Winkel: full-time employee of Ferring B.V., The Netherlands.
Figures
References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49(6):1374–1403. - PubMed
-
- Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: A 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int 2008;102(11):1531–1538. - PubMed
-
- Tombal B, Miller K, Boccon-Gibod L, et al. Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur Urol 2010;57(5):836–842. - PubMed
-
- Schröder FH, Tombal B, Miller K, et al. Changes in alkaline phosphatase levels in patients with prostate cancer receiving degarelix or leuprolide: Results from a 12-month, comparative, phase III study. BJU Int 2010;106(2):182–187. - PubMed
-
- Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol 2014;65(3):565–573. - PubMed
LinkOut - more resources
Full Text Sources