Roles of N-Methyl-D-Aspartate Receptors (NMDARs) in Epilepsy
- PMID: 35069111
- PMCID: PMC8780133
- DOI: 10.3389/fnmol.2021.797253
Roles of N-Methyl-D-Aspartate Receptors (NMDARs) in Epilepsy
Abstract
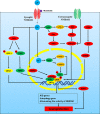
Epilepsy is one of the most common neurological disorders characterized by recurrent seizures. The mechanism of epilepsy remains unclear and previous studies suggest that N-methyl-D-aspartate receptors (NMDARs) play an important role in abnormal discharges, nerve conduction, neuron injury and inflammation, thereby they may participate in epileptogenesis. NMDARs belong to a family of ionotropic glutamate receptors that play essential roles in excitatory neurotransmission and synaptic plasticity in the mammalian CNS. Despite numerous studies focusing on the role of NMDAR in epilepsy, the relationship appeared to be elusive. In this article, we reviewed the regulation of NMDAR and possible mechanisms of NMDAR in epilepsy and in respect of onset, development, and treatment, trying to provide more evidence for future studies.
Keywords: CREB; D-serine; N-methyl-D-aspartate receptor; anti-NMDAR encephalitis; epigenomics; epilepsy; excitotoxicity; glutamate.
Copyright © 2022 Chen, Xu, Fan, Fang, Wang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

