The Gut-Brain Axis and Its Relation to Parkinson's Disease: A Review
- PMID: 35069178
- PMCID: PMC8776990
- DOI: 10.3389/fnagi.2021.782082
The Gut-Brain Axis and Its Relation to Parkinson's Disease: A Review
Abstract
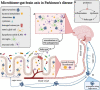
Parkinson's disease is a chronic neurodegenerative disease characterized by the accumulation of misfolded alpha-synuclein protein (Lewy bodies) in dopaminergic neurons of the substantia nigra and other related circuitry, which contribute to the development of both motor (bradykinesia, tremors, stiffness, abnormal gait) and non-motor symptoms (gastrointestinal issues, urinogenital complications, olfaction dysfunction, cognitive impairment). Despite tremendous progress in the field, the exact pathways and mechanisms responsible for the initiation and progression of this disease remain unclear. However, recent research suggests a potential relationship between the commensal gut bacteria and the brain capable of influencing neurodevelopment, brain function and health. This bidirectional communication is often referred to as the microbiome-gut-brain axis. Accumulating evidence suggests that the onset of non-motor symptoms, such as gastrointestinal manifestations, often precede the onset of motor symptoms and disease diagnosis, lending support to the potential role that the microbiome-gut-brain axis might play in the underlying pathological mechanisms of Parkinson's disease. This review will provide an overview of and critically discuss the current knowledge of the relationship between the gut microbiota and Parkinson's disease. We will discuss the role of α-synuclein in non-motor disease pathology, proposed pathways constituting the connection between the gut microbiome and the brain, existing evidence related to pre- and probiotic interventions. Finally, we will highlight the potential opportunity for the development of novel preventative measures and therapeutic options that could target the microbiome-gut-brain axis in the context of Parkinson's disease.
Keywords: Parkinson’s disease; alpha-synuclein; dysbiosis; gut–brain axis; microbiome.
Copyright © 2022 Klann, Dissanayake, Gurrala, Farrer, Shukla, Ramirez-Zamora, Mai and Vedam-Mai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Acupuncture modulates the microbiota-gut-brain axis: a new strategy for Parkinson's disease treatment.Front Aging Neurosci. 2025 Aug 7;17:1640389. doi: 10.3389/fnagi.2025.1640389. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40851666 Free PMC article. Review.
-
Exploring the role of gut microbiota in Parkinson's disease: insights from fecal microbiota transplantation.Front Neurosci. 2025 Jun 13;19:1574512. doi: 10.3389/fnins.2025.1574512. eCollection 2025. Front Neurosci. 2025. PMID: 40584885 Free PMC article. Review.
-
Gut microbiota and Parkinson's Disease: a new frontier in understanding neurological health.Inflammopharmacology. 2025 May;33(5):2565-2588. doi: 10.1007/s10787-025-01726-w. Epub 2025 Apr 17. Inflammopharmacology. 2025. PMID: 40244491 Review.
-
Tissue Factor and Its Cerebrospinal Fluid Protein Profiles in Parkinson's Disease.J Parkinsons Dis. 2024;14(7):1405-1416. doi: 10.3233/JPD-240115. J Parkinsons Dis. 2024. PMID: 39240648 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
Cited by
-
Oral and gut microbiome profiles in people with early idiopathic Parkinson's disease.Commun Med (Lond). 2024 Oct 23;4(1):209. doi: 10.1038/s43856-024-00630-8. Commun Med (Lond). 2024. PMID: 39443634 Free PMC article.
-
The pathobiological basis of depression in Parkinson disease: challenges and outlooks.J Neural Transm (Vienna). 2022 Dec;129(12):1397-1418. doi: 10.1007/s00702-022-02559-5. Epub 2022 Nov 2. J Neural Transm (Vienna). 2022. PMID: 36322206 Free PMC article. Review.
-
Changes in Bacterial Gut Composition in Parkinson's Disease and Their Metabolic Contribution to Disease Development: A Gut Community Reconstruction Approach.Microorganisms. 2024 Feb 4;12(2):325. doi: 10.3390/microorganisms12020325. Microorganisms. 2024. PMID: 38399728 Free PMC article.
-
Intestinal Barrier Dysfunction in the Absence of Systemic Inflammation Fails to Exacerbate Motor Dysfunction and Brain Pathology in a Mouse Model of Parkinson's Disease.Front Neurol. 2022 May 18;13:882628. doi: 10.3389/fneur.2022.882628. eCollection 2022. Front Neurol. 2022. PMID: 35665034 Free PMC article.
-
The microbiota-gut-brain axis and three common neurological disorders: a mini-review.Ann Med Surg (Lond). 2023 Apr 1;85(5):1780-1783. doi: 10.1097/MS9.0000000000000552. eCollection 2023 May. Ann Med Surg (Lond). 2023. PMID: 37228957 Free PMC article.
References
-
- Alipour Nosrani E., Tamtaji O. R., Alibolandi Z., Sarkar P., Ghazanfari M., Azami Tameh A., et al. (2021). Neuroprotective effects of probiotics bacteria on animal model of Parkinson’s disease induced by 6-hydroxydopamine: A behavioral, biochemical, and histological study. J. Immunoassay Immunochem. 42 106–120. 10.1080/15321819.2020.1833917 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

