theLiTE™: A Screening Platform to Identify Compounds that Reinforce Tight Junctions
- PMID: 35069190
- PMCID: PMC8771259
- DOI: 10.3389/fphar.2021.752787
theLiTE™: A Screening Platform to Identify Compounds that Reinforce Tight Junctions
Abstract
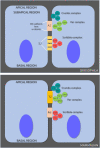
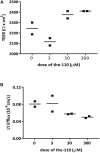
Tight junctions (TJ) are formed by transmembrane and intracellular proteins that seal the intercellular space and control selective permeability of epithelia. Integrity of the epithelial barrier is central to tissue homeostasis and barrier dysfunction has been linked to many pathological conditions. TJ support the maintenance of cell polarity through interactions with the Par complex (Cdc42-Par-6-Par-3-aPKC) in which Par-6 is an adaptor and links the proteins of the complex together. Studies have shown that Par-6 overexpression delays the assembly of TJ proteins suggesting that Par-6 negatively regulates TJ assembly. Because restoring barrier integrity is of key therapeutic and prophylactic value, we focus on finding compounds that have epithelial barrier reinforcement properties; we developed a screening platform (theLiTE™) to identify compounds that modulate Par-6 expression in follicular epithelial cells from Par-6-GFP Drosophila melanogaster egg chambers. Hits identified were then tested whether they improve epithelial barrier function, using measurements of transepithelial electrical resistance (TEER) or dye efflux to evaluate paracellular permeability. We tested 2,400 compounds, found in total 10 hits. Here we present data on six of them: the first four hits allowed us to sequentially build confidence in theLiTE™ and two compounds that were shortlisted for further development (myricetin and quercetin). We selected quercetin due to its clinical and scientific validation as a compound that regulates TJ; food supplement formulated on the basis of this discovery is currently undergoing clinical evaluation in gastroesophageal reflux disease (GERD) sufferers.
Keywords: PAR-6; epithelial cells; myricetin; polarity (cell); quercetin; tight junctions.
Copyright © 2022 Gomes, Oliveira-Marques, Hampson, Jacinto, de Moraes and Martinho.
Conflict of interest statement
LM and RH were employed by Thelial Technologies during all or part of the work on this study. As such the funder had involvement in study design, collection, analysis, interpretation of data and the writing of this article. The decision to submit it for publication was taken independently. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
-
- Amanzadeh E., Esmaeili A., Abadi R. E. N., Kazemipour N., Pahlevanneshan Z., Beheshti S. (2019). Quercetin Conjugated with Superparamagnetic Iron Oxide Nanoparticles Improves Learning and Memory Better Than Free Quercetin via Interacting with Proteins Involved in LTP. Sci. Rep. 9 (1), 6876. 10.1038/s41598-019-43345-w - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

