Insights on Cancer Cell Inhibition, Subcellular Activities, and Kinase Profile of Phenylacetamides Pending 1 H-Imidazol-5-One Variants
- PMID: 35069208
- PMCID: PMC8766756
- DOI: 10.3389/fphar.2021.794325
Insights on Cancer Cell Inhibition, Subcellular Activities, and Kinase Profile of Phenylacetamides Pending 1 H-Imidazol-5-One Variants
Abstract
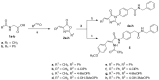
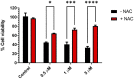
Structural changes of small-molecule drugs may bring interesting biological properties, especially in the field of kinase inhibitors. We sought to study tirbanibulin, a first-in-class dual Src kinase (non-ATP competitive)/tubulin inhibitor because there was not enough reporting about its structure-activity relationships (SARs). In particular, the present research is based on the replacement of the outer ring of the biphenyl system of 2-[(1,1'-biphenyl)-4-yl]-N-benzylacetamide, the identified pharmacophore of KX chemotype, with a heterocyclic ring. The newly synthesized compounds showed a range of activities in cell-based anticancer assays, agreeing with a clear SAR profile. The most potent compound, (Z)-N-benzyl-4-[4-(4-methoxybenzylidene)-2-methyl-5-oxo-4,5-dihydro-1H-imidazol-1-yl]phenylacetamide (KIM-161), demonstrated cytotoxic IC50 values at 294 and 362 nM against HCT116 colon cancer and HL60 leukemia cell lines, respectively. Profiling of this compound (aqueous solubility, liver microsomal stability, cytochrome P450 inhibition, reactivity with reduced glutathione, and plasma protein binding) confirmed its adequate drug-like properties. Mechanistic studies revealed that this compound does not depend on tubulin or Src kinase inhibition as a factor in forcing HL60 to exit its cell cycle and undergo apoptosis. Instead, KIM-161 downregulated several other kinases such as members of BRK, FLT, and JAK families. It also strongly suppresses signals of ERK1/2, GSK-3α/β, HSP27, and STAT2, while it downregulated AMPKα1 phosphorylation within the HL60 cells. Collectively, these results suggest that phenylacetamide-1H-imidazol-5-one (KIM-161) could be a promising lead compound for further clinical anticancer drug development.
Keywords: imidazolone; leukemia; multi-kinase downregulation; phosphokinase profiling; scaffold hopping; src kinase; structure–properties relationship; tirbanibulin.
Copyright © 2022 Khayat, Omar, Ahmed, Khan, Ibrahim, Muhammad, Malebari, Neamatallah and El-Araby.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Anbalagan M., Carrier L., Glodowski S., Hangauer D., Shan B., Rowan B. G. (2012). KX-01, a Novel Src Kinase Inhibitor Directed toward the Peptide Substrate Site, Synergizes with Tamoxifen in Estrogen Receptor α Positive Breast Cancer. Breast Cancer Res. Treat. 132, 391–409. 10.1007/s10549-011-1513-3 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

