Real-World Evidence for the Safety and Efficacy of CGRP Monoclonal Antibody Therapy Added to OnabotulinumtoxinA Treatment for Migraine Prevention in Adult Patients With Chronic Migraine
- PMID: 35069416
- PMCID: PMC8770868
- DOI: 10.3389/fneur.2021.788159
Real-World Evidence for the Safety and Efficacy of CGRP Monoclonal Antibody Therapy Added to OnabotulinumtoxinA Treatment for Migraine Prevention in Adult Patients With Chronic Migraine
Abstract
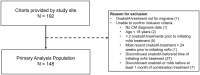
Background: OnabotulinumtoxinA and calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) target different migraine pathways, therefore, combination treatment may provide additional effectiveness for the preventive treatment of chronic migraine (CM) than either treatment alone. The objective of this study was to collect real-world data to improve the understanding of the safety, tolerability, and effectiveness of adding a CGRP mAb to onabotulinumtoxinA treatment for the preventive treatment of CM. Methods: This was a retrospective, longitudinal study conducted using data extracted from a single clinical site's electronic medical records (EMR) of adult patients (≥18 years) with CM treated with ≥2 consecutive cycles of onabotulinumtoxinA before ≥1 month of continuous onabotulinumtoxinA and CGRP mAb (erenumab, fremanezumab, or galcanezumab) combination treatment. Safety was evaluated by the rate of adverse events (AE) and serious adverse events (SAE). The proportion of patients who discontinued either onabotulinumtoxinA, a CGRP mAb, or combination treatment, and the reason for discontinuation, if available, was collected. The effectiveness of combination preventive treatment was assessed by the reduction in monthly headache days (MHD). Outcome data were extracted from EMR at the first CGRP mAb prescription (index) and up to four assessments at ~3, 6, 9, and 12 months post-index. The final analyses were based on measures consistently reported in the EMR. Results: EMR were collected for 192 patients, of which 148 met eligibility criteria and were included for analysis. Erenumab was prescribed to 56.7% of patients, fremanezumab to 42.6%, and galcanezumab to 0.7%. Mean (standard deviation [SD]) MHD were 20.4 (6.6) prior to onabotulinumtoxinA treatment and 14.0 (6.9) prior to the addition of a CGRP mAb (baseline). After real-world addition of a CGRP mAb, there were significant reductions in MHD at the first assessment (~3 months) (mean -2.6 days/month, 95% CI -3.7, -1.4) and at all subsequent visits. After ~12 months of continuous combination treatment, MHD were reduced by 4.6 days/month (95% CI -6.7, -2.5) and 34.9% of patients achieved ≥50% MHD reduction from index. AEs were reported by 18 patients (12.2%), with the most common being constipation (n = 8, 5.4% [onabotulinumtoxinA plus erenumab only]) and injection site reactions (n = 5, 3.4%). No SAEs were reported. Overall, 90 patients (60.8%) discontinued one or both treatments. The most common reason for discontinuing either treatment was lack of insurance coverage (40%); few (~14%) patients discontinued a CGRP mAb and none discontinued onabotulinumtoxinA due to safety/tolerability. Conclusion: In this real-world study, onabotulinumtoxinA was effective at reducing MHD and the addition of a CGRP mAb was safe, well-tolerated and associated with incremental and clinically meaningful reductions in MHD for those who stayed on the combination treatment. No new safety signals were identified. Of those who discontinued, the majority reported lack of insurance coverage as a reason. Prospective real-world and controlled trials are needed to further evaluate the safety and potential benefits of this combination treatment paradigm for people with CM.
Keywords: calcitonin gene-related peptide; combination treatment; headache; migraine; onabotulinumtoxinA; prevention; safety.
Copyright © 2022 Mechtler, Saikali, McVige, Hughes, Traut and Adams.
Conflict of interest statement
LM has received personal compensation for consulting, serving on a scientific advisory board, speaking, research affiliation, or other activities with Alder Pharmaceuticals, Allergan (now AbbVie Inc.), Amgen, Avanir, Biohaven, Boston Biomedical Inc., CellDex, DelMar Pharmaceuticals, electroCore, Novartis, Orbis Pharmaceuticals, Promius, Teva Pharmaceuticals, and Jushi; he has financial interest in Jushi. NS has served as speaker and/or advisory board member for Allergan (now AbbVie Inc.), Amgen, Avanir, Biohaven, Currax, Depomed, Egalet, GammaCore, Eli Lilly, Lundbeck, Pernix, Promius, Supernus, and Teva. JM has served as a speaker and/or received research support from Allergan (now AbbVie Inc.), Amgen/Novartis, Avanir, Biohaven, Eli Lilly, Lundbeck, Theranica, Amneal and Teva. OH is an employee of ICON plc. AT and AA are full-time employees of AbbVie Inc., and may hold AbbVie stock. The authors declare that this study received funding from Allergan, an AbbVie Company. Employees of AbbVie participated in the research, interpretation of data, review of the manuscript, and the decision to submit for publication.
Figures




Similar articles
-
Real-World Evidence for Control of Chronic Migraine Patients Receiving CGRP Monoclonal Antibody Therapy Added to OnabotulinumtoxinA: A Retrospective Chart Review.Pain Ther. 2021 Dec;10(2):809-826. doi: 10.1007/s40122-021-00264-x. Epub 2021 Apr 21. Pain Ther. 2021. PMID: 33880725 Free PMC article. Review.
-
Real-world effectiveness of Anti-CGRP monoclonal antibodies compared to OnabotulinumtoxinA (RAMO) in chronic migraine: a retrospective, observational, multicenter, cohort study.J Headache Pain. 2024 Feb 2;25(1):14. doi: 10.1186/s10194-024-01721-6. J Headache Pain. 2024. PMID: 38308209 Free PMC article.
-
Persistence to anti-CGRP monoclonal antibodies and onabotulinumtoxinA among patients with migraine: a retrospective cohort study.J Headache Pain. 2023 Aug 2;24(1):101. doi: 10.1186/s10194-023-01636-8. J Headache Pain. 2023. PMID: 37532991 Free PMC article.
-
Comparative effectiveness and tolerability of calcitonin gene-related peptide (CGRP) monoclonal antibodies and onabotulinumtoxinA in chronic migraine: A multicenter, real-world study in Taiwan.Eur J Neurol. 2024 Sep;31(9):e16372. doi: 10.1111/ene.16372. Epub 2024 Jun 5. Eur J Neurol. 2024. PMID: 38837528 Free PMC article.
-
Monoclonal antibodies blocking CGRP transmission: An update on their added value in migraine prevention.Rev Neurol (Paris). 2020 Dec;176(10):788-803. doi: 10.1016/j.neurol.2020.04.027. Epub 2020 Aug 2. Rev Neurol (Paris). 2020. PMID: 32758365 Review.
Cited by
-
Real-world effectiveness, satisfaction, and optimization of ubrogepant for the acute treatment of migraine in combination with onabotulinumtoxinA: results from the COURAGE Study.J Headache Pain. 2023 Aug 3;24(1):102. doi: 10.1186/s10194-023-01622-0. J Headache Pain. 2023. PMID: 37537578 Free PMC article.
-
Combining onabotulinumtoxin A with a CGRP antagonist for chronic migraine prophylaxis: where do we stand?Front Pain Res (Lausanne). 2023 Oct 27;4:1292994. doi: 10.3389/fpain.2023.1292994. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37965209 Free PMC article. No abstract available.
-
Combining treatments for migraine prophylaxis: the state-of-the-art.J Headache Pain. 2024 Dec 5;25(1):214. doi: 10.1186/s10194-024-01925-w. J Headache Pain. 2024. PMID: 39639191 Free PMC article. Review.
-
Monoclonal Antibodies against Calcitonin Gene-Related Peptide for Migraine Prophylaxis: A Systematic Review of Real-World Data.Cells. 2022 Dec 29;12(1):143. doi: 10.3390/cells12010143. Cells. 2022. PMID: 36611935 Free PMC article.
-
Onabotulinumtoxin-A: Previous Prophylactic Treatment Might Improve Subsequent Anti-CGRP Monoclonal Antibodies Response in Patients with Chronic Migraine.Toxins (Basel). 2023 Nov 30;15(12):677. doi: 10.3390/toxins15120677. Toxins (Basel). 2023. PMID: 38133181 Free PMC article.
References
-
- Lanteri-Minet M, Duru G, Mudge M, Cottrell S. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia. (2011) 31:837–50. 10.1177/0333102411398400 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

