TGF-β1 Drives Inflammatory Th Cell But Not Treg Cell Compartment Upon Allergen Exposure
- PMID: 35069535
- PMCID: PMC8777012
- DOI: 10.3389/fimmu.2021.763243
TGF-β1 Drives Inflammatory Th Cell But Not Treg Cell Compartment Upon Allergen Exposure
Abstract
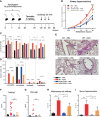
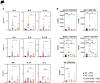
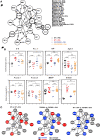
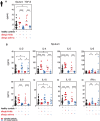
TGF-β1 is known to have a pro-inflammatory impact by inducing Th9 and Th17 cells, while it also induces anti-inflammatory Treg cells (Tregs). In the context of allergic airway inflammation (AAI) its dual role can be of critical importance in influencing the outcome of the disease. Here we demonstrate that TGF-β is a major player in AAI by driving effector T cells, while Tregs differentiate independently. Induction of experimental AAI and airway hyperreactivity in a mouse model with inducible genetic ablation of the gene encoding for TGFβ-receptor 2 (Tgfbr2) on CD4+T cells significantly reduced the disease phenotype. Further, it blocked the induction of pro-inflammatory T cell frequencies (Th2, Th9, Th17), but increased Treg cells. To translate these findings into a human clinically relevant context, Th2, Th9 and Treg cells were quantified both locally in induced sputum and systemically in blood of allergic rhinitis and asthma patients with or without allergen-specific immunotherapy (AIT). Natural allergen exposure induced local and systemic Th2, Th9, and reduced Tregs cells, while therapeutic allergen exposure by AIT suppressed Th2 and Th9 cell frequencies along with TGF-β and IL-9 secretion. Altogether, these findings support that neutralization of TGF-β represents a viable therapeutic option in allergy and asthma, not posing the risk of immune dysregulation by impacting Tregs cells.
Keywords: TGF-beta; Th17; Th2; Th9; allergen-specific immunotherapy; asthma; induced sputum.
Copyright © 2021 Musiol, Alessandrini, Jakwerth, Chaker, Schneider, Guerth, Schnautz, Grosch, Ghiordanescu, Ullmann, Kau, Plaschke, Haak, Buch, Schmidt-Weber and Zissler.
Conflict of interest statement
UZ received payment for manuscripts from Deutsches Aerzteblatt and funds for travel from the European Academy of Allergy and Clinical Immunology (EAACI) and Collegium Internationale Allergologicum (CIA). CS-W received support for research projects from PLS Design, LETI, Zeller AG, and Allergopharma and accepted honoraria for consultancy and seminars from LETI and Allergopharma. He also received travel support from EAACI. AC has consultant arrangements through Technical University Munich with Allergopharma, ALK-Abello, HAL Allergy, Mundipharma, and Lofarma; has conducted clinical studies and received research grants through Technical University Munich from Allergopharma, Novartis, the German Federal Environmental Agency, Bencard/Allergen Therapeutics, ASIT Biotech, and Zeller AG; has received payment for lectures from Allergopharma, ALK-Abello, and GlaxoSmithKline; has received payment for manuscript preparation from Bayerisches Ärzteblatt; and has received travel support from the European Academy of Allergy and Clinical Immunology (EAACI), DGAKI, and SMI. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

