Complement Inhibition Alleviates Cholestatic Liver Injury Through Mediating Macrophage Infiltration and Function in Mice
- PMID: 35069557
- PMCID: PMC8777082
- DOI: 10.3389/fimmu.2021.785287
Complement Inhibition Alleviates Cholestatic Liver Injury Through Mediating Macrophage Infiltration and Function in Mice
Abstract
Background and aims: Cholestatic liver injury (CLI), which is associated with inflammatory reactions and oxidative stress, is a serious risk factor for postoperative complications. Complement system is involved in a wide range of liver disorders, including cholestasis. The present study assessed the role of complement in CLI and the therapeutic effect of the site-targeted complement inhibitor CR2-Crry in CLI.
Methods: Wild-type and complement gene deficient mice underwent common bile duct ligation (BDL) to induce CLI or a sham operation, followed by treatment with CR2-Crry or GdCl3. The roles of complement in CLI and the potential therapeutic effects of CR2-Crry were investigated by biochemical analysis, flow cytometry, immunohistochemistry, ELISA, and quantitative RT-PCR.
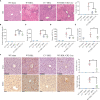
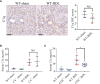
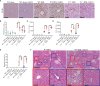
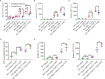
Results: C3 deficiency and CR2-Crry significantly reduced liver injuries in mice with CLI, and also markedly decreasing the numbers of neutrophils and macrophages in the liver. C3 deficiency and CR2-Crry also significantly reduced neutrophil expression of Mac-1 and liver expression of VCAM-1. More importantly, C3 deficiency and CR2-Crry significantly inhibited M1 macrophage polarization in these mice. Intravenous injection of GdCl3 inhibited macrophage infiltration and activation in the liver. However, the liver injury increased significantly. BDL significantly increased the level of lipopolysaccharide (LPS) in portal blood, but not in peripheral blood. GdCl3 significantly increased LPS in peripheral blood, suggesting that macrophages clear portal blood LPS. Oral administration of ampicillin to in GdCl3 treated mice reduced LPS levels in portal blood and alleviated liver damage. In contrast, intraperitoneal injection LPS increased portal blood LPS and reversed the protective effect of ampicillin. Interestingly, C3 deficiency did not affect the clearance of LPS.
Conclusions: Complement is involved in CLI, perhaps mediating the infiltration and activation of neutrophils and macrophage M1 polarization in the liver. C3 deficiency and CR2-Crry significantly alleviated CLI. Inhibition of complement could preserve the protective function of macrophages in clearing LPS, suggesting that complement inhibition could be useful in treating CLI.
Keywords: CR2-Crry; cholestatic liver injury; complement system; macrophage; neutrophil.
Copyright © 2022 Guo, Chen, Zeng, Wang, Yao, Tomlinson, Chen, Yuan and He.
Conflict of interest statement
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A complement C3 inhibitor specifically targeted to sites of complement activation effectively ameliorates collagen-induced arthritis in DBA/1J mice.J Immunol. 2007 Dec 1;179(11):7860-7. doi: 10.4049/jimmunol.179.11.7860. J Immunol. 2007. PMID: 18025232
-
Targeted Complement Inhibition Protects Vascularized Composite Allografts From Acute Graft Injury and Prolongs Graft Survival When Combined With Subtherapeutic Cyclosporine A Therapy.Transplantation. 2017 Apr;101(4):e75-e85. doi: 10.1097/TP.0000000000001625. Transplantation. 2017. PMID: 28045880 Free PMC article.
-
A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice.J Clin Invest. 2009 Aug;119(8):2304-16. doi: 10.1172/JCI38289. Epub 2009 Jul 20. J Clin Invest. 2009. PMID: 19620784 Free PMC article.
-
Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice.J Immunol. 2008 Jan 15;180(2):1231-8. doi: 10.4049/jimmunol.180.2.1231. J Immunol. 2008. PMID: 18178863
-
Complement plays an important role in spinal cord injury and represents a therapeutic target for improving recovery following trauma.Am J Pathol. 2006 Sep;169(3):1039-47. doi: 10.2353/ajpath.2006.060248. Am J Pathol. 2006. PMID: 16936276 Free PMC article.
Cited by
-
Cholestasis-induced phenotypic transformation of neutrophils contributes to immune escape of colorectal cancer liver metastasis.J Biomed Sci. 2024 Jun 29;31(1):66. doi: 10.1186/s12929-024-01052-3. J Biomed Sci. 2024. PMID: 38951890 Free PMC article.
-
Association of complement components with the risk and severity of NAFLD: A systematic review and meta-analysis.Front Immunol. 2022 Dec 7;13:1054159. doi: 10.3389/fimmu.2022.1054159. eCollection 2022. Front Immunol. 2022. PMID: 36569882 Free PMC article.
-
Exercise preconditioning and resveratrol reduce the susceptibility of rats with obstructive jaundice to endotoxin and alleviate lung injury.Front Immunol. 2024 Dec 18;15:1466615. doi: 10.3389/fimmu.2024.1466615. eCollection 2024. Front Immunol. 2024. PMID: 39744639 Free PMC article.
-
Inflammasome and pyroptosis in autoimmune liver diseases.Front Immunol. 2023 Mar 8;14:1150879. doi: 10.3389/fimmu.2023.1150879. eCollection 2023. Front Immunol. 2023. PMID: 36969233 Free PMC article. Review.
-
The Role of Inflammation in Cholestatic Liver Injury.J Inflamm Res. 2023 Oct 13;16:4527-4540. doi: 10.2147/JIR.S430730. eCollection 2023. J Inflamm Res. 2023. PMID: 37854312 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

