Induction of Functional Specific Antibodies, IgG-Secreting Plasmablasts and Memory B Cells Following BCG Vaccination
- PMID: 35069580
- PMCID: PMC8767055
- DOI: 10.3389/fimmu.2021.798207
Induction of Functional Specific Antibodies, IgG-Secreting Plasmablasts and Memory B Cells Following BCG Vaccination
Abstract
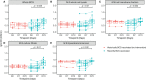
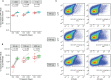
Tuberculosis (TB) is a major global health problem and the only currently-licensed vaccine, BCG, is inadequate. Many TB vaccine candidates are designed to be given as a boost to BCG; an understanding of the BCG-induced immune response is therefore critical, and the opportunity to relate this to circumstances where BCG does confer protection may direct the design of more efficacious vaccines. While the T cell response to BCG vaccination has been well-characterized, there is a paucity of literature on the humoral response. We demonstrate BCG vaccine-mediated induction of specific antibodies in different human populations and macaque species which represent important preclinical models for TB vaccine development. We observe a strong correlation between antibody titers in serum versus plasma with modestly higher titers in serum. We also report for the first time the rapid and transient induction of antibody-secreting plasmablasts following BCG vaccination, together with a robust and durable memory B cell response in humans. Finally, we demonstrate a functional role for BCG vaccine-induced specific antibodies in opsonizing mycobacteria and enhancing macrophage phagocytosis in vitro, which may contribute to the BCG vaccine-mediated control of mycobacterial growth observed. Taken together, our findings indicate that the humoral immune response in the context of BCG vaccination merits further attention to determine whether TB vaccine candidates could benefit from the induction of humoral as well as cellular immunity.
Keywords: B cells; BCG; TB; antibodies; humoral immunity; tuberculosis; vaccine.
Copyright © 2022 Bitencourt, Peralta-Álvarez, Wilkie, Jacobs, Wright, Salman Almujri, Li, Harris, Smith, Elias, White, Satti, Sharpe, O’Shea, McShane and Tanner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- WHO . World Health Organisation Global Tuberculosis Report 2020. Geneva:World Health Organization; (2020).
-
- Orme IM. Characteristics and Specificity of Acquired Immunologic Memory to Mycobacterium Tuberculosis Infection. J Immunol (1988) 140(10):3589–93. - PubMed
-
- Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice Deficient in CD4 T Cells Have Only Transiently Diminished Levels of IFN-Gamma, Yet Succumb to Tuberculosis. J Immunol (1999) 162(9):5407–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

