Single-Cell RNA Profiling of Human Skin Reveals Age-Related Loss of Dermal Sheath Cells and Their Contribution to a Juvenile Phenotype
- PMID: 35069694
- PMCID: PMC8776708
- DOI: 10.3389/fgene.2021.797747
Single-Cell RNA Profiling of Human Skin Reveals Age-Related Loss of Dermal Sheath Cells and Their Contribution to a Juvenile Phenotype
Abstract
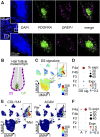
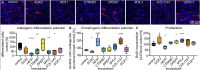
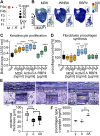
The dermal sheath (DS) is a population of mesenchyme-derived skin cells with emerging importance for skin homeostasis. The DS includes hair follicle dermal stem cells, which exhibit self-renewal and serve as bipotent progenitors of dermal papilla (DP) cells and DS cells. Upon aging, stem cells exhibit deficiencies in self-renewal and their number is reduced. While the DS of mice has been examined in considerable detail, our knowledge of the human DS, the pathways contributing to its self-renewal and differentiation capacity and potential paracrine effects important for tissue regeneration and aging is very limited. Using single-cell RNA sequencing of human skin biopsies from donors of different ages we have now analyzed the transcriptome of 72,048 cells, including 50,149 fibroblasts. Our results show that DS cells that exhibit stem cell characteristics were lost upon aging. We further show that HES1, COL11A1, MYL4 and CTNNB1 regulate DS stem cell characteristics. Finally, the DS secreted protein Activin A showed paracrine effects on keratinocytes and dermal fibroblasts, promoting proliferation, epidermal thickness and pro-collagen production. Our work provides a detailed description of human DS identity on the single-cell level, its loss upon aging, its stem cell characteristics and its contribution to a juvenile skin phenotype.
Keywords: Activin A; aging; dermal sheath cells; fibroblasts; regeneration; single-cell RNA sequencing; skin; stem cells.
Copyright © 2022 Ahlers, Falckenhayn, Holzscheck, Solé-Boldo, Schütz, Wenck, Winnefeld, Lyko, Grönniger and Siracusa.
Conflict of interest statement
Authors JMDA, CF, NH, HW, MW, EG and AS are employed by the Beiersdorf AG. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F. C., Krause D. S., et al. (2006). Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 8, 315–317. 10.1080/14653240600855905 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

