Role of Metabolic Reprogramming of Long non-coding RNA in Clear Cell Renal Cell Carcinoma
- PMID: 35069912
- PMCID: PMC8771523
- DOI: 10.7150/jca.62683
Role of Metabolic Reprogramming of Long non-coding RNA in Clear Cell Renal Cell Carcinoma
Abstract
Renal cell carcinoma (RCC), one of the most frequent cancers, is a "classical" malignancy characterized by metabolic reprogramming. Clear cell renal cell carcinoma (ccRCC) is its most common histopathological subtype. Long-stranded non-coding ribonucleic acids (LncRNAs) are regulatory RNA molecules with limited protein-coding capacity and evolutionary conservation. Recent studies have revealed that lncRNAs can broadly regulate the metabolic reprogramming of ccRCC and its malignant transformation. However, there are few studies on lncRNAs regulating the metabolism of ccRCC, and the specific mechanisms are unknown. Therefore, this paper summarizes the regulatory mechanisms of lncRNAs in the metabolism of ccRCC, especially in the pathways of glycolysis, mitochondrial function, glutamine and lipid metabolism, cellular mechanisms, interactions with other molecules, specific scientific and clinic implications and applications to provide a basis for early clinical diagnosis, prediction and treatment. We also discuss the clinical application and challenges of targeting lncRNAs in ccRCC metabolism.
Keywords: LncRNAs; metabolic reprogramming; renal clear cell carcinoma.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

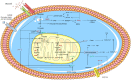
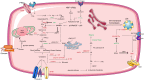
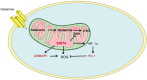

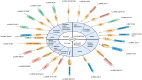
References
-
- Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Moch H, Cubilla AL, Humphrey PA. et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93–105. - PubMed
-
- Chen LL. Linking Long Noncoding RNA Localization and Function. Trends Biochem Sci. 2016;41:761–772. - PubMed
-
- Lucarelli G, Loizzo D, Franzin R. et al. Metabolomic insights into pathophysiological mechanisms and biomarker discovery in clear cell renal cell carcinoma. Expert Rev Mol Diagn. 2019;19:397–407. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

