Hepatoid esophagogastric adenocarcinoma and tumoral heterogeneity: a case report
- PMID: 35070435
- PMCID: PMC8748022
- DOI: 10.21037/jgo-21-287
Hepatoid esophagogastric adenocarcinoma and tumoral heterogeneity: a case report
Abstract
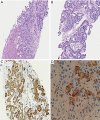
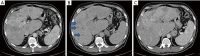
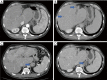
Hepatoid adenocarcinoma of the stomach is an uncommon subtype of gastric cancer remarkably similar to hepatocellular carcinoma in histopathological analysis. It is also commonly associated with high serum alfa-fetoprotein and a poorer prognosis, despite the emergence of new therapeutic options. In recent years, next generation sequencing (NGS) technology has made it possible to identify and describe the genes and molecular alterations common to gastric cancer thereby contributing to the advancement of targeted therapies. A 62-year-old patient, with no prior risk factor for hepatocellular carcinoma (HCC), presented to the emergency room with dysphagia for solids, abdominal pain and weight loss of about 3 kilograms over 3 months. Histopathological analysis presented with disparities regarding HER2 and programmed death-ligand 1 (PD-L1) status in the primary and metastatic sites. We describe a case of a de novo metastatic, human epidermal growth factor receptor 2 (HER2) positive esophagogastric junction hepatoid adenocarcinoma. Although this is a rare subgroup of gastric cancer, treatment strategies were based in recent studies in immunotherapy and guided therapy, taking into consideration the molecular findings from the patient's tumor NGS analysis. Data about HER2 and PDL1 heterogeneity were also reviewed. Despite the aggressiveness and rarity of this histology, the patient had a good response to treatment.
Keywords: Case report; esophagogastric junction; hepatoid adenocarcinoma; heterogeneity; human epidermal growth factor receptor 2 (HER2).
2021 Journal of Gastrointestinal Oncology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-287). The series “Educational Case Series of the Memorial Sloan Kettering Cancer Center” was commissioned by the editorial office without any sponsorship or funding. TBC received support from Astra Zeneca, Bayer, Eli Lilly, Genentech/Roche, Ipsen, Merck KGaA, Merck Sharp & Dohme. AS received support from Roche, Merck, BMS, Sanofi, Eli Lilly and is a consultant to Roche, MSD, Merck, Sanofi, Astellas and Johnson & Johnson, and he received honoraria for lectures and presentation from Roche, Merck, BMS, MSD, Johnson & Johnson and Amgen and support for attending meetings from Merck. GMB receives research funding to his institution from mAbxience, Merck Sharp & Dohme Corp. and Bristol-Myers Squibb. DM received research support from Astellas, Pfizer, and Astra Zeneca, and honoraria/travel support from MSD, BMS, Astellas, Bayer and Janssen. GdSF received honoraria/travel support from BMS, MSD, Roche and Ipsen and advisory board support from BMS, MSD, Boeringher, Sanofi, Roche, Astelas, Bayer. GKA served as the unpaid Guest Editor for the series and he received research support from Arcus, Agios, Astra Zeneca, BioNtech, BMS, Celgene, Flatiron, Genentech/Roche, Genoscience, Incyte, Polaris, Puma, QED, Silenseed, Yiviva, and consulting support for efforts with Adicet, Astra Zeneca, Alnylam, Autem, Bayer, Beigene, Berry Genomics, Cend, Celgene, CytomX, Eisai, Eli Lilly, Exelixis, Flatiron, Genentech/Roche, Genoscience, Helio, Incyte, Ipsen, Legend Biotech, Merck, Nerviano, QED, Redhill, Rafael, Servier, Silenseed, Sillajen, Sobi, Surface Oncology, Therabionics, Vector, and Yiviva. YYJ received research support from Bayer, Bristol-Myers Squibb, Cycle for Survival, Department of Defense, Eli Lilly, Fred’s Team, Genentech/Roche, Merck, NCI, RGENIX and receives consulting fees from Astra Zeneca, Basilea Pharmaceutica, Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Imugene, Merck, Merck Serono, Michael J. Hennessy Associates, Paradigm Medical Communications, Pfizer, RGENIX, Seagen, Zymeworks Inc., and has Stock Options of RGENIX. The authors have no other conflict of interest to report.
Figures

References
-
- Yano T, Kishimoto T, Tomaru U, et al. Further evidence of hepatic transdifferentiation in hepatoid adenocarcinomas of the stomach: quantitative analysis of mRNA for albumin and hepatocyte nuclear factor-4alpha. Pathology 2003;35:75-8. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
