Use of adjuvant chemotherapy in resected non-small cell lung cancer in real-life practice: a systematic review of literature
- PMID: 35070767
- PMCID: PMC8743521
- DOI: 10.21037/tlcr-21-557
Use of adjuvant chemotherapy in resected non-small cell lung cancer in real-life practice: a systematic review of literature
Abstract
Background: Adjuvant chemotherapy (AC) is recommended since 2004 for patients with a completely resected non-small cell lung cancer (NSCLC). Indeed, several randomized clinical trials have demonstrated an improved survival for patients treated with adjuvant cisplatin-based regimen than surgery alone. In these large clinical trials, patients were well selected and fit to receive AC. As the benefit of AC was estimated at 5.4% of 5-year overall survival (OS), it seems important to evaluate AC use in a less selected population. In particular, elderly patients were underrepresented in large randomized clinical trials. Furthermore, other confounding factors might limit AC efficacy in real-life practice such as the delay of chemotherapy initiation following lung surgery or the number of AC cycles received. Therefore, the aim of this systematic review is to summarize the state of the literature on AC use in current clinical practice.
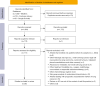
Methods: A systematic assessment of literature articles and reviews on AC use in real-life practice was performed by searching in several relevant database including Medline, Google Scholar and Cochrane Library following PICOS (i.e., Population, Intervention, Comparison, Outcomes, Study design) eligibility criteria and PRISMA guidelines. Among the 1,957 results obtained with the request formulated on these research database, 56 relevant articles on AC use in non-trial setting were selected and included in the results section.
Results: This systematic literature review highlights the lack of literature on AC use in real-life practice as most of these studies were retrospective. Interestingly, a delayed AC-mostly due to postoperative complications-was better than surgery alone. Furthermore, AC was less purposed to elderly patients, despite retrospective studies outlined that this therapeutic option could be benefit in this specific population as for younger patients. In real-life practice, AC was also often incomplete due to adverse events, but dose reduction or omission was not always associated with an inferior survival. In non-trial setting, number of AC cycles delivered, dose reduction or omission is quite similar to randomized clinical trials.
Discussion: Nowadays, AC is part of the therapeutic strategy used in completely resected NSCLC. In a population of less selected patients, this systematic literature review shows that AC can be used safely and efficiently, especially in elderly patients. As well, delayed AC seems effective. Finally, the place of immunotherapy and targeted therapies have to be precised in the future as well as biomarkers to better select patients that would response to chemotherapy.
Keywords: 8th TNM classification; Adjuvant chemotherapy (AC); non-small cell lung cancer (NSCLC); stage IIA to IIIA.
2021 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-557). The authors have no conflicts of interest to declare.
Figures
References
-
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. Erratum in: Lancet Oncol 2006;7:797. 10.1016/S1470-2045(06)70804-X - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials