A Systematic Review and Meta-Analysis of the Effectiveness of Neuroprotectants for Paclitaxel-Induced Peripheral Neuropathy
- PMID: 35070969
- PMCID: PMC8766304
- DOI: 10.3389/fonc.2021.763229
A Systematic Review and Meta-Analysis of the Effectiveness of Neuroprotectants for Paclitaxel-Induced Peripheral Neuropathy
Abstract
Background: Paclitaxel-induced peripheral neuropathy (PIPN) is a disabling side effect of paclitaxel with few effective preventive strategies. We aim to determine the efficacy of pharmacological and non-pharmacological neuroprotective interventions in preventing PIPN incidence.
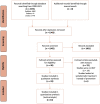
Methods: Biomedical literature databases were searched from years 2000 to 2021 for trials comparing neuroprotective interventions and control. Meta-analysis was performed using the random-effects model. The primary outcome was the incidence of PIPN.
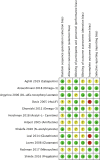
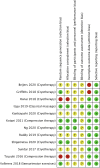
Results: Of 24 relevant controlled trials, 14 were eligible for meta-analysis. Pooled results from seven non-pharmacological trials were associated with a statistically significant 48% relative reduction of PIPN risk with low heterogeneity. Conversely, pooled results from six pharmacological trials were associated with a significant 20% relative reduction of PIPN risk with moderate heterogeneity. Both pharmacological and non-pharmacological approaches appear effective in reducing PIPN incidence in the treatment arm compared to control (pooled RR < 1).
Conclusion: Current evidence suggests that both interventions may reduce PIPN risk. Non-pharmacological interventions appear more effective than pharmacological interventions.
Keywords: chemotherapy-induced peripheral neuropathy (CIPN); neuroprotection; non-invasive; paclitaxel-induced peripheral neuropathy; prevention; taxane.
Copyright © 2022 Leen, Yap, Teo, Tan, Molassiotis, Ishiguro, Fan, Sundar, Soon and Bandla.
Conflict of interest statement
RS has received honoraria from Bristol-Myers Squibb, Lilly, Roche, Taiho, Astra Zeneca, DKSH, and MSD; has advisory activity with Bristol-Myers Squibb, Merck, Eisai, Bayer, Taiho, Novartis, MSD, and AstraZeneca; received research funding from MSD and Paxman Coolers; and has received travel grants from AstraZeneca, Eisai, Roche, and Taiho Pharmaceutical. AB reports grants from Paxman Coolers Ltd., outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
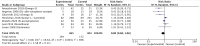

References
-
- Loprinzi CL. Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy. In: UpToDate (2017). Available at: https://www.uptodate.com/contents/prevention-and-treatment-of-chemothera....
-
- Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. . Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2014) 32(18):1941–67. doi: 10.1200/JCO.2013.54.0914 - DOI - PubMed
-
- Molassiotis A, Cheng HL, Lopez V, Au JS, Chan A, Bandla A, et al. . Are We Mis-Estimating Chemotherapy-Induced Peripheral Neuropathy? Analysis of Assessment Methodologies From a Prospective, Multinational, Longitudinal Cohort Study of Patients Receiving Neurotoxic Chemotherapy. BMC Cancer (2019) 19(1):132. doi: 10.1186/s12885-019-5302-4 - DOI - PMC - PubMed
-
- Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB. Healthcare Costs and Workloss Burden of Patients With Chemotherapy-Associated Peripheral Neuropathy in Breast, Ovarian, Head and Neck, and Nonsmall Cell Lung Cancer. Chemother Res Pract (2012) 2012:913848. doi: 10.1155/2012/913848 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

