Intra-Species Interactions in Streptococcus pneumoniae Biofilms
- PMID: 35071049
- PMCID: PMC8767070
- DOI: 10.3389/fcimb.2021.803286
Intra-Species Interactions in Streptococcus pneumoniae Biofilms
Abstract
Streptococcus pneumoniae is a human pathogen responsible for high morbidity and mortality worldwide. Disease is incidental and is preceded by asymptomatic nasopharyngeal colonization in the form of biofilms. Simultaneous colonization by multiple pneumococcal strains is frequent but remains poorly characterized. Previous studies, using mostly laboratory strains, showed that pneumococcal strains can reciprocally affect each other's colonization ability. Here, we aimed at developing a strategy to investigate pneumococcal intra-species interactions occurring in biofilms. A 72h abiotic biofilm model mimicking long-term colonization was applied to study eight pneumococcal strains encompassing 6 capsular types and 7 multilocus sequence types. Strains were labeled with GFP or RFP, generating two fluorescent variants for each. Intra-species interactions were evaluated in dual-strain biofilms (1:1 ratio) using flow cytometry. Confocal microscopy was used to image representative biofilms. Twenty-eight dual-strain combinations were tested. Interactions of commensalism, competition, amensalism and neutralism were identified. The outcome of an interaction was independent of the capsular and sequence type of the strains involved. Confocal imaging of biofilms confirmed the positive, negative and neutral effects that pneumococci can exert on each other. In conclusion, we developed an experimental approach that successfully discriminates pneumococcal strains growing in mixed biofilms, which enables the identification of intra-species interactions. Several types of interactions occur among pneumococci. These observations are a starting point to study the mechanisms underlying those interactions.
Keywords: Streptococcus pneumoniae; biofilms; co-colonization; colonization; competition; intraspecies interactions; multiple carriage.
Copyright © 2022 Valente, Cruz, Henriques and Sá-Leão.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
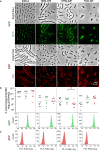

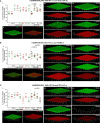
References
-
- Avery O. T., Macleod C. M., Mccarty M. (1944). Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types : Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated From Pneumococcus Type Iii. J. Exp. Med. 79, 137–158. doi: 10.1084/jem.79.2.137 - DOI - PMC - PubMed
-
- Barnes D. M., Whittier S., Gilligan P. H., Soares S., Tomasz A., Henderson F. W. (1995). Transmission of Multidrug-Resistant Serotype 23F Streptococcus Pneumoniae in Group Day Care: Evidence Suggesting Capsular Transformation of the Resistant Strain In Vivo . J. Infect. Dis. 171, 890–896. doi: 10.1093/infdis/171.4.890 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

