Molecular Mechanisms of Staphylococcus and Pseudomonas Interactions in Cystic Fibrosis
- PMID: 35071057
- PMCID: PMC8770549
- DOI: 10.3389/fcimb.2021.824042
Molecular Mechanisms of Staphylococcus and Pseudomonas Interactions in Cystic Fibrosis
Abstract
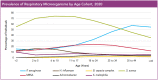
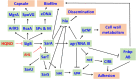
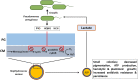
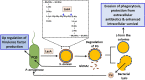
Cystic fibrosis (CF) is an autosomal recessive genetic disorder that is characterized by recurrent and chronic infections of the lung predominantly by the opportunistic pathogens, Gram-positive Staphylococcus aureus and Gram-negative Pseudomonas aeruginosa. While S. aureus is the main colonizing bacteria of the CF lungs during infancy and early childhood, its incidence declines thereafter and infections by P. aeruginosa become more prominent with increasing age. The competitive and cooperative interactions exhibited by these two pathogens influence their survival, antibiotic susceptibility, persistence and, consequently the disease progression. For instance, P. aeruginosa secretes small respiratory inhibitors like hydrogen cyanide, pyocyanin and quinoline N-oxides that block the electron transport pathway and suppress the growth of S. aureus. However, S. aureus survives this respiratory attack by adapting to respiration-defective small colony variant (SCV) phenotype. SCVs cause persistent and recurrent infections and are also resistant to antibiotics, especially aminoglycosides, antifolate antibiotics, and to host antimicrobial peptides such as LL-37, human β-defensin (HBD) 2 and HBD3; and lactoferricin B. The interaction between P. aeruginosa and S. aureus is multifaceted. In mucoid P. aeruginosa strains, siderophores and rhamnolipids are downregulated thus enhancing the survival of S. aureus. Conversely, protein A from S. aureus inhibits P. aeruginosa biofilm formation while protecting both P. aeruginosa and S. aureus from phagocytosis by neutrophils. This review attempts to summarize the current understanding of the molecular mechanisms that drive the competitive and cooperative interactions between S. aureus and P. aeruginosa in the CF lungs that could influence the disease outcome.
Keywords: Pseudomonas aeruginosa; Staphylococcus aureus; adaptation; antagonism; co-existence; cystic fibrosis.
Copyright © 2022 Biswas and Götz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Andrey D. O., Jousselin A., Villanueva M., Renzoni A., Monod A., Barras C., et al. . (2015). Impact of the Regulators SigB, Rot, SarA and sarS on the Toxic Shock Tst Promoter and TSST-1 Expression in Staphylococcus Aureus . PloS One 10, e0135579. doi: 10.1371/journal.pone.0135579 - DOI - PMC - PubMed
-
- Baldan R., Cigana C., Testa F., Bianconi I., De Simone M., Pellin D., et al. . (2014). Adaptation of Pseudomonas Aeruginosa in Cystic Fibrosis Airways Influences Virulence of Staphylococcus Aureus In Vitro and Murine Models of Co-Infection. PloS One 9, e89614. doi: 10.1371/journal.pone.0089614 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

