Review on Strategies and Technologies for Exosome Isolation and Purification
- PMID: 35071216
- PMCID: PMC8766409
- DOI: 10.3389/fbioe.2021.811971
Review on Strategies and Technologies for Exosome Isolation and Purification
Abstract
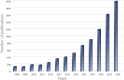
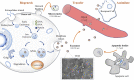
Exosomes, a nano-sized subtype of extracellular vesicles secreted from almost all living cells, are capable of transferring cell-specific constituents of the source cell to the recipient cell. Cumulative evidence has revealed exosomes play an irreplaceable role in prognostic, diagnostic, and even therapeutic aspects. A method that can efficiently provide intact and pure exosomes samples is the first step to both exosome-based liquid biopsies and therapeutics. Unfortunately, common exosomal separation techniques suffer from operation complexity, time consumption, large sample volumes and low purity, posing significant challenges for exosomal downstream analysis. Efficient, simple, and affordable methods to isolate exosomes are crucial to carrying out relevant researches. In the last decade, emerging technologies, especially microfluidic chips, have proposed superior strategies for exosome isolation and exhibited fascinating performances. While many excellent reviews have overviewed various methods, a compressive review including updated/improved methods for exosomal isolation is indispensable. Herein, we first overview exosomal properties, biogenesis, contents, and functions. Then, we briefly outline the conventional technologies and discuss the challenges of clinical applications of these technologies. Finally, we review emerging exosomal isolation strategies and large-scale GMP production of engineered exosomes to open up future perspectives of next-generation Exo-devices for cancer diagnosis and treatment.
Keywords: cancer; exosome isolation; exosome separation; exosomes; microfluidics.
Copyright © 2022 Chen, Li, Zhang, Xu, Huang, Wang and Du.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

