Ultra-high-throughput Ca2+ assay in platelets to distinguish ITAM-linked and G-protein-coupled receptor activation
- PMID: 35072010
- PMCID: PMC8762394
- DOI: 10.1016/j.isci.2021.103718
Ultra-high-throughput Ca2+ assay in platelets to distinguish ITAM-linked and G-protein-coupled receptor activation
Abstract
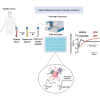
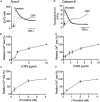
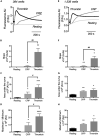
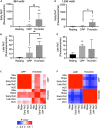
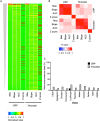
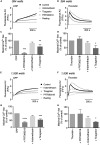
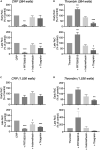
Antiplatelet drugs targeting G-protein-coupled receptors (GPCRs), used for the secondary prevention of arterial thrombosis, coincide with an increased bleeding risk. Targeting ITAM-linked receptors, such as the collagen receptor glycoprotein VI (GPVI), is expected to provide a better antithrombotic-hemostatic profile. Here, we developed and characterized an ultra-high-throughput (UHT) method based on intracellular [Ca2+]i increases to differentiate GPVI and GPCR effects on platelets. In 96-, 384-, or 1,536-well formats, Calcium-6-loaded human platelets displayed a slow-prolonged or fast-transient [Ca2+]i increase when stimulated with the GPVI agonist collagen-related peptide or with thrombin and other GPCR agonists, respectively. Semi-automated curve fitting revealed five parameters describing the Ca2+ responses. Verification of the UHT assay was done with a robustness compound library and clinically relevant platelet inhibitors. Taken together, these results present proof of principle of distinct receptor-type-dependent Ca2+ signaling curves in platelets, which allow identification of new inhibitors in a UHT way.
Keywords: Cell biology; Functional aspects of cell biology; Methodology in biological sciences.
© 2021 The Author(s).
Conflict of interest statement
J.W.M.H. is a cofounder and shareholder of FlowChamber B.V. The other authors declare no relevant conflicts of interest.
Figures

Similar articles
-
Ultra-high throughput-based screening for the discovery of antiplatelet drugs affecting receptor dependent calcium signaling dynamics.Sci Rep. 2024 Mar 14;14(1):6229. doi: 10.1038/s41598-024-56799-4. Sci Rep. 2024. PMID: 38486006 Free PMC article.
-
Platelet calcium signaling by G-protein coupled and ITAM-linked receptors regulating anoctamin-6 and procoagulant activity.Platelets. 2021 Oct 3;32(7):863-871. doi: 10.1080/09537104.2020.1859103. Epub 2020 Dec 26. Platelets. 2021. PMID: 33356720 Review.
-
High-throughput assessment identifying major platelet Ca2+ entry pathways via tyrosine kinase-linked and G protein-coupled receptors.Cell Calcium. 2023 Jun;112:102738. doi: 10.1016/j.ceca.2023.102738. Epub 2023 Apr 8. Cell Calcium. 2023. PMID: 37060673
-
Redundancy and interaction of thrombin- and collagen-mediated platelet activation in tail bleeding and carotid thrombosis in mice.Arterioscler Thromb Vasc Biol. 2014 Dec;34(12):2563-9. doi: 10.1161/ATVBAHA.114.304244. Epub 2014 Oct 2. Arterioscler Thromb Vasc Biol. 2014. PMID: 25278288 Free PMC article.
-
Glycoprotein VI interplay with fibrin(ogen) in thrombosis.J Thromb Haemost. 2023 Jul;21(7):1703-1713. doi: 10.1016/j.jtha.2023.03.022. Epub 2023 Mar 28. J Thromb Haemost. 2023. PMID: 36990158 Review.
Cited by
-
Challenges and future perspectives for high-throughput chimeric antigen receptor T cell discovery.Curr Opin Biotechnol. 2024 Dec;90:103216. doi: 10.1016/j.copbio.2024.103216. Epub 2024 Oct 21. Curr Opin Biotechnol. 2024. PMID: 39437676 Review.
-
Platelet Functional Testing Via High-Throughput Microtiter Plate-Based Assays.Curr Protoc. 2023 Feb;3(2):e668. doi: 10.1002/cpz1.668. Curr Protoc. 2023. PMID: 36786557 Free PMC article.
-
Platelet Hemostasis Reactions at Different Temperatures Correlate with Intracellular Calcium Concentration.Int J Mol Sci. 2022 Sep 14;23(18):10667. doi: 10.3390/ijms231810667. Int J Mol Sci. 2022. PMID: 36142580 Free PMC article.
-
Ultra-high throughput-based screening for the discovery of antiplatelet drugs affecting receptor dependent calcium signaling dynamics.Sci Rep. 2024 Mar 14;14(1):6229. doi: 10.1038/s41598-024-56799-4. Sci Rep. 2024. PMID: 38486006 Free PMC article.
-
Suppressed ORAI1-STIM1-dependent Ca2+ entry by protein kinase C isoforms regulating platelet procoagulant activity.J Biol Chem. 2024 Dec;300(12):107899. doi: 10.1016/j.jbc.2024.107899. Epub 2024 Oct 17. J Biol Chem. 2024. PMID: 39424145 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

