Hyperglycemia Induces Meibomian Gland Dysfunction
- PMID: 35072689
- PMCID: PMC8802017
- DOI: 10.1167/iovs.63.1.30
Hyperglycemia Induces Meibomian Gland Dysfunction
Abstract
Purpose: Patients diagnosed with diabetes are inclined to have abnormalities on stability of tear film and disorder of meibomian gland (MG). This study aims to explore the pathological change of MG induced by diabetes in a rat model.
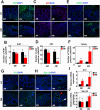
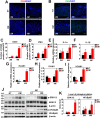
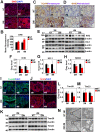
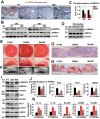
Methods: Sprague-Dawley (SD) rats were intraperitoneally injected with streptozotocin (STZ) to establish a diabetic animal model. Lipid accumulation in MG was detected by Oil Red O staining and LipidTox staining. Cell proliferation status was determined by Ki67 and P63 immunostaining, whereas cell apoptosis was confirmed by TUNEL assay. Gene expression of inflammatory cytokines and adhesion molecules IL-1α, IL-1β, ELAM1, ICAM1, and VCAM1 were detected by RT-PCR. Activation of ERK, NF-κB, and AMPK signaling pathways was determined by Western Blot analysis. Oxidative stress-related factors NOX4, 4HNE, Nrf2, HO-1, and SOD2 were detected by immunostaining or Western Blot analysis. Tom20 and Tim23 immunostaining and transmission electron microscopy were performed to evaluate the mitochondria functional and structure change.
Results: Four months after STZ injection, there was acini dropout in MG of diabetic rats. Evident infiltration of inflammatory cells, increased expression of inflammatory factors, and adhesion molecules, as well as activated ERK and NF-κB signaling pathways were identified. Oxidative stress of MG was evident in 4-month diabetic rats. Phospho-AMPK was downregulated in MG of 2-month diabetic rats and more prominent in 4-month rats. After metformin treatment, phospho-AMPK was upregulated and the morphology of MG was well maintained. Moreover, inflammation and oxidative stress of MG were alleviated after metformin intervention.
Conclusions: Long-term diabetes may lead to Meibomian gland dysfunction (MGD). AMPK may be a therapeutic target of MGD induced by diabetes.
Conflict of interest statement
Disclosure:
Figures


References
-
- Jester JV, Nicolaides N, Smith RE.. Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci. 1981; 20: 537–547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

