Ex vivo propagation in a novel 3D high-throughput co-culture system for multiple myeloma
- PMID: 35072775
- PMCID: PMC9016043
- DOI: 10.1007/s00432-021-03854-6
Ex vivo propagation in a novel 3D high-throughput co-culture system for multiple myeloma
Abstract
Purpose: Multiple myeloma (MM) remains an incurable hematologic malignancy which ultimately develops drug resistance and evades treatment. Despite substantial therapeutic advances over the past years, the clinical failure rate of preclinically promising anti-MM drugs remains substantial. More realistic in vitro models are thus required to better predict clinical efficacy of a preclinically active compound.
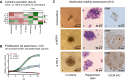
Methods: Here, we report on the establishment of a conical agarose 3D co-culture platform for the preclinical propagation of primary MM cells ex vivo. Cell growth was compared to yet established 2D and liquid overlay systems. MM cell lines (MMCL: RPMI-8226, U266, OPM-2) and primary patient specimens were tested. Drug sensitivity was examined by exploring the cytotoxic effect of bortezomib and the deubiquitinase inhibitor auranofin under various conditions.
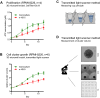
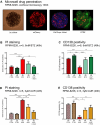
Results: In contrast to 2D and liquid overlay, cell proliferation in the 3D array followed a sigmoidal curve characterized by an initial growth delay but more durable proliferation of MMCL over 12 days of culture. Primary MM specimens did not expand in ex vivo monoculture, but required co-culture support by a human stromal cell line (HS-5, MSP-1). HS-5 induced a > fivefold increase in cluster volume and maintained long-term viability of primary MM cells for up to 21 days. Bortezomib and auranofin induced less cytotoxicity under 3D vs. 2D condition and in co- vs. monoculture, respectively.
Conclusions: This study introduces a novel model that is capable of long-term propagation and drug testing of primary MM specimens ex vivo overcoming some of the pitfalls of currently available in vitro models.
Keywords: Auranofin; Bone marrow microenvironment; Bortezomib; Drug discovery; In vitro modeling; Multiple myeloma.
© 2022. The Author(s).
Conflict of interest statement
A.R.T.: Inventor of 3D CoSeedis™ (patent #EP2917326A1 and #WO2014072432A1); consultant to abc biopply. 3D CoSeedis™ is commercially licensed by abc biopply, Cham, Switzerland. All other authors report no conflict of interest with regard to this publication.
Figures


References
-
- Abe M, Hiura K, Wilde J et al (2004) Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood 104:2484–2491. 10.1182/blood-2003-11-3839 - PubMed
-
- American Cancer Society. Key statistics for multiple myeloma. https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html. Accessed 13 June 2021
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

