Pickering Interfacial Catalysis for Aerobic Alcohol Oxidation in Oil Foams
- PMID: 35073074
- PMCID: PMC8815424
- DOI: 10.1021/jacs.1c11207
Pickering Interfacial Catalysis for Aerobic Alcohol Oxidation in Oil Foams
Abstract
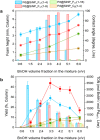
Oil foams stabilized by surface-active catalytic particles bearing fluorinated chains and Pd nanoparticles allowed fast and efficient aerobic oxidation of a variety of aromatic and aliphatic alcohols compared to bulk catalytic systems at ambient O2 pressure. High foam stability was achieved at low particle concentration (<1 wt %) provided that the contact angle locates in the range 41°-73°. The catalytic performance was strongly affected by the foaming properties, with 7-10 times activity increase in pure O2 compared to nonfoam systems. Intermediate foam stability was required to achieve good catalytic activity, combining large interfacial area and high gas exchange rate. Particles were conveniently recycled with high foamability and catalytic efficiency maintained for at least seven consecutive runs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Shah Y. T.Gas-Liquid-Solid Reactor Design; McGraw-Hill: New York, 1979.
- Ramachandran P. A.; Chaudari R. V.. Three-Phase Catalytic Reactors; Topics in Chemical Engineering; Gordon and Breach Science Publishers: Philadelphia, 1983; Vol. 2, Chapter 9.
- Trambouze P.; Euzen J. P.. Chemical Reactors: From Design to Operation; Editions Technip: Paris, 2004.
- Henkel K.-D.Reactor Types and Their Industrial Applications. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, 2012; Vol. 31, pp 293–327.
-
- Kobayashi J.; Mori Y.; Okamoto K.; Akiyama R.; Ueno M.; Kitamori T.; Kobayashi S. A Microfluidic Device for Conducting Gas-Liquid-Solid Hydrogenation Reactions. Science 2004, 304, 1305–1308. 10.1126/science.1096956. - DOI - PubMed
- Jähnisch K.; Hessel V.; Lowe H.; Baerns M. Chemistry in Microstructured Reactors. Angew. Chem., Int. Ed. 2004, 43, 406.10.1002/anie.200300577. - DOI - PubMed
- Hessel V.; Angeli P.; Gavriilidis A.; Löwe H. Gas-Liquid and Gas-Liquid-Solid Microstructured Reactors: Contacting Principles and Applications. Ind. Eng. Chem. Res. 2005, 44, 9750–9769. 10.1021/ie0503139. - DOI
- Irfan M.; Glasnov T. N.; Kappe C. O. Heterogeneous Catalytic Hydrogenation Reactions in Continuous-Flow Reactors. ChemSusChem 2011, 4, 300–316. 10.1002/cssc.201000354. - DOI - PubMed
- Dencic I.; Hessel V.; de Croon M. H. J. M.; Meuldijk J.; van der Doelen C. W. J.; Koch K. Recent Changes in Patenting Behavior in Microprocess Technology and its Possible Use for Gas-Liquid Reactions and the Oxidation of Glucose. ChemSusChem 2012, 5, 232–245. 10.1002/cssc.201100389. - DOI - PubMed
- Brzozowski M.; O’Brien M.; Ley S. V.; Polyzos A. Flow Chemistry: Intelligent Processing of Gas-Liquid Transformations Using a Tube-in-Tube Reactor. Acc. Chem. Res. 2015, 48, 349–362. 10.1021/ar500359m. - DOI - PubMed
- Gutmann B.; Cantillo D.; Kappe C. O. Continuous-Flow Technology – a Tool for the Safe Manufacturing of Active Pharmaceutical Ingredients. Angew. Chem., Int. Ed. 2015, 54, 6688–6728. 10.1002/anie.201409318. - DOI - PubMed
- Yeong K. K.; Gavriilidis A.; Zapf R.; Hessel V. Catalyst Preparation and Deactivation Issues for Nitrobenzene Hydrogenation in a Microstructured Falling Film Reactor. Catal. Today 2003, 81, 641–651. 10.1016/S0920-5861(03)00162-7. - DOI
- Yeong K. K.; Gavriilidis A.; Zapf R.; Hessel V. Experimental Studies of Nitrobenzene Hydrogenation in a Microstructured Falling Film. Reactor. Chem. Eng. Sci. 2004, 59, 3491–3494. 10.1016/j.ces.2004.04.022. - DOI
-
- Ræder H.; Bredesen R.; Crehan G.; Miachon S.; Dalmon J.-A.; Pintar A.; Levec J.; Torp E. G. Wet Air Oxidation Process Using a Catalytic Membrane Contactor. Sep. Purif. Technol. 2003, 32, 349–355. 10.1016/S1383-5866(03)00056-X. - DOI
- Reif M.; Dittmeyer R. Porous, Catalytically Active Ceramic Membranes for Gas-Liquid Reactions: a Comparison Between Catalytic Diffuser and Forced Through Flow Concept. Catal. Today 2003, 82, 3–14. 10.1016/S0920-5861(03)00197-4. - DOI
- Miachon S.; Dalmon J.-A. Catalysis in Membrane Reactors: What About the Catalyst?. Top. Catal. 2004, 29, 59–65. 10.1023/B:TOCA.0000024928.63811.8b. - DOI
-
- Pera-Titus M.; Leclercq L.; Clacens J. M.; De Campo F.; Nardello-Rataj V. Pickering Interfacial Catalysis for Biphasic Systems: From Emulsion Design to Green Reactions. Angew. Chem., Int. Ed. 2015, 54, 2006–2021. 10.1002/anie.201402069. - DOI - PubMed
- Bago Rodriguez A. M.; Binks B. P. Catalysis in Pickering Emulsions. Soft Matter 2020, 16, 10221–10243. 10.1039/D0SM01636E. - DOI - PubMed
-
- Drenckhan W. Generation of Superstable, Monodisperse Microbubbles Using a pH-Driven Assembly of Surface-Active Particles. Angew. Chem., Int. Ed. 2009, 48, 5245–5347. 10.1002/anie.200901531. - DOI - PubMed
- Horozov T. S. Foams and Foam Films Stabilised by Solid Particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. 10.1016/j.cocis.2007.11.009. - DOI
- Paunov V. N.; Al-Shehri H.; Horozov T. S. Attachment of Composite Porous Supra-Particles to Air-Water and Oil-Water Interfaces: Theory and Experiment. Phys. Chem. Chem. Phys. 2016, 18, 26495–26508. 10.1039/C6CP05453F. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

