AT-752, a double prodrug of a guanosine nucleotide analog, inhibits yellow fever virus in a hamster model
- PMID: 35073319
- PMCID: PMC8812913
- DOI: 10.1371/journal.pntd.0009937
AT-752, a double prodrug of a guanosine nucleotide analog, inhibits yellow fever virus in a hamster model
Abstract
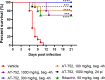
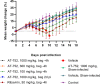
Yellow fever virus (YFV) is a zoonotic pathogen re-emerging in parts of the world, causing a viral hemorrhagic fever associated with high mortality rates. While an effective vaccine is available, having an effective antiviral against YFV is critical against unexpected outbreaks, or when vaccination is not recommended. We have previously identified AT-281, the free base of AT-752, an orally available double prodrug of a guanosine nucleotide analog, as a potent inhibitor of YFV in vitro, with a 50% effective concentration (EC50) of 0.31 μM. In hamsters infected with YFV (Jimenez strain), viremia rose about 4 log10-fold and serum alanine aminotransferase (ALT) 2-fold compared to sham-infected animals. Treatment with 1000 mg/kg AT-752 for 7 days, initiated 4 h prior to viral challenge, reduced viremia to below the limit of detection by day 4 post infection (pi) and returned ALT to normal levels by day 6 pi. When treatment with AT-752 was initiated 2 days pi, the virus titer and ALT dropped >2 log10 and 53% by day 4 and 6 pi, respectively. In addition, at 21 days pi, 70-100% of the infected animals in the treatment groups survived compared to 0% of the untreated group (p<0.001). Moreover, in vivo formation of the active triphosphate metabolite AT-9010 was measured in the animal tissues, with the highest concentrations in liver and kidney, organs that are vulnerable to the virus. The demonstrated in vivo activity of AT-752 suggests that it is a promising compound for clinical development in the treatment of YFV infection.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: KL, AM and JS are employees of Atea Pharmaceuticals, Inc. and SG is a consultant for Atea Pharmaceuticals, Inc.
Figures




References
-
- Soteras Jalil E. Yellow Fever. World Health Organization [Internet] [updated 7 May 2019; cited 2021 8 Jun]. Available from: https://www.who.int/news-room/fact-sheets/detail/yellow-fever.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

