Immune and Metabolic Effects of Antigen-Specific Immunotherapy Using Multiple β-Cell Peptides in Type 1 Diabetes
- PMID: 35073398
- PMCID: PMC8965665
- DOI: 10.2337/db21-0728
Immune and Metabolic Effects of Antigen-Specific Immunotherapy Using Multiple β-Cell Peptides in Type 1 Diabetes
Abstract
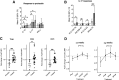
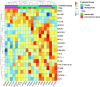
Type 1 diabetes is characterized by a loss of tolerance to pancreatic β-cell autoantigens and defects in regulatory T-cell (Treg) function. In preclinical models, immunotherapy with MHC-selective, autoantigenic peptides restores immune tolerance, prevents diabetes, and shows greater potency when multiple peptides are used. To translate this strategy into the clinical setting, we administered a mixture of six HLA-DRB1*0401-selective, β-cell peptides intradermally to patients with recent-onset type 1 diabetes possessing this genotype in a randomized placebo-controlled study at monthly doses of 10, 100, and 500 μg for 24 weeks. Stimulated C-peptide (measuring insulin functional reserve) had declined in all placebo subjects at 24 weeks but was maintained at ≥100% baseline levels in one-half of the treated group. Treatment was accompanied by significant changes in islet-specific immune responses and a dose-dependent increase in Treg expression of the canonical transcription factor FOXP3 and changes in Treg gene expression. In this first-in-human study, multiple-peptide immunotherapy shows promise as a strategy to correct immune regulatory defects fundamental to the pathobiology of autoimmune diabetes.
Trial registration: ClinicalTrials.gov NCT02620332.
© 2022 by the American Diabetes Association.
Conflict of interest statement
Figures
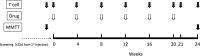

Comment in
-
Comment on Liu et al. Immune and Metabolic Effects of Antigen-Specific Immunotherapy Using Multiple β-Cell Peptides in Type 1 Diabetes. Diabetes 2022;71:722-732.Diabetes. 2022 Dec 1;71(12):e20-e21. doi: 10.2337/db22-0584. Diabetes. 2022. PMID: 36409789 No abstract available.
References
-
- Patterson CC, Gyürüs E, Rosenbauer J, et al. . Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia 2012;55:2142–2147 - PubMed
-
- Killestein J. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;347:1116–1117 - PubMed
-
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. . Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

