Growth pattern trajectories in boys with Duchenne muscular dystrophy
- PMID: 35073949
- PMCID: PMC8785507
- DOI: 10.1186/s13023-021-02158-9
Growth pattern trajectories in boys with Duchenne muscular dystrophy
Abstract
Objectives: The objective of this study is to analyse retrospective, observational, longitudinal growth (weight, height and BMI) data in ambulatory boys aged 5-12 years with Duchenne muscular dystrophy (DMD).
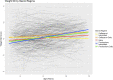
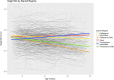
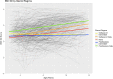
Background: We considered glucocorticoids (GC) use, dystrophin isoforms and amenability to exon 8, 44, 45, 51 and 53 skipping drug subgroups, and the impact of growth on loss of ambulation. We analysed 598 boys, with 2604 observations. This analysis considered patients from the UK NorthStar database (2003-2020) on one of five regimes: "GC naïve", "deflazacort daily" (DD), "deflazacort intermittent" (DI), "prednisolone daily" (PD) and "prednisolone intermittent" (PI). A random slope model was used to model the weight, height and BMI SD scores (using the UK90).
Results: The daily regime subgroups had significant yearly height stunting compared to the GC naïve subgroup. Notably, the average height change for the DD subgroup was 0.25 SD (95% CI - 0.30, - 0.21) less than reference values. Those with affected expression of Dp427, Dp140 and Dp71 isoforms were 0.77 (95% CI 0.3, 1.24) and 0.82 (95% CI 1.28, 0.36) SD shorter than those with Dp427 and/or Dp140 expression affected respectively. Increased weight was not associated with earlier loss of ambulation, but taller boys still ambulant between the age of 10 and 11 years were more at risk of losing ambulation.
Conclusion: These findings may provide further guidance to clinicians when counselling and discussing GCs commencement with patients and their carers and may represent a benchmark set of data to evaluate the effects of new generations of GC.
Keywords: Deflazacort; Duchenne muscular dystrophy; Glucocorticoids; Growth; Isoforms; Prednisolone.
© 2022. The Author(s).
Conflict of interest statement
GS, SR, MC, MF, DR, VAG, RDA, AS and AM have no conflict of interest. FM is supported by the NIHR Great Ormond Street Hospital Biomedical Research Centre and has received speaker and consultancy honoraria from Sarepta Therapeutics, Avexis, PTC Therapeutics, Roche and Pfizer. The views expressed in this paper are his and not necessarily those of the NHS, NIHR or the department of health. GB is PI of clinical trials Sponsored by Pfizer, NS Pharma, and Reveragen, and has received speaker and/or consulting fees from Sarepta, PTC Therapeutics, Biogen, Novartis Gene Therapies, Inc. (AveXis), and Roche and has worked as principal investigator of SMA studies sponsored by Novartis Gene Therapies, Inc., and Roche.
Figures
References
-
- Wagner KR, Kuntz NL, Koenig E, East L, Upadhyay S, Han B, et al. Safety, tolerability, and pharmacokinetics of casimersen in patients with Duchenne muscular dystrophy amenable to exon 45 skipping: a randomized, double-blind, placebo-controlled, dose-titration trial. Muscle Nerve. 2021;64(3):285–292. doi: 10.1002/mus.27347. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous