Intravenous vitamin C administration to patients with septic shock: a pilot randomised controlled trial
- PMID: 35073968
- PMCID: PMC8786621
- DOI: 10.1186/s13054-022-03900-w
Intravenous vitamin C administration to patients with septic shock: a pilot randomised controlled trial
Abstract
Background: Intravenous vitamin C administration in septic shock may have a sparing effect on vasopressor requirements, and vitamin C's enzyme cofactor functions provide a mechanistic rationale. Our study aimed to determine the effect of intravenous vitamin C administration on vasopressor requirements and other outcomes in patients with septic shock.
Methods: This was a double-blind, randomised placebo-controlled trial in 40 patients with septic shock who were randomised (1:1) to receive intravenous vitamin C (at a dose of 25 mg/kg of body weight every 6 h) or placebo (intravenous 5% dextrose) for up to 96 h, or until death or discharge. The primary outcome was intravenous vasopressor requirements (dose and duration), and secondary outcomes included Sequential Organ Failure Assessment (SOFA) scores, intensive care unit (ICU) and hospital length of stay, and mortality. In addition, blood samples were collected to determine vitamin C kinetics and inflammatory marker concentrations.
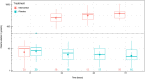
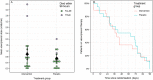
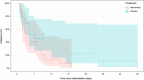
Results: Median plasma vitamin C concentrations were deficient at baseline (9.2 [4.4, 12] µmol/L) and increased to 408 (227, 560) µmol/L following 72 h of intervention. The mean duration of intravenous vasopressor infusion in the vitamin C group was 48 (95% CI 35-62) hours and in the placebo group was 54 (95% CI 41-62) hours (p = 0.52). The dose of vasopressor delivered over time was comparable between the two groups, as were SOFA scores (p > 0.05). The median ICU length of stay in the intervention group was 3.8 (2.2, 9.8) days versus 7.1 (3.1, 20) days in the placebo group (p = 0.12). The median hospital length of stay for the vitamin C group was 18 (11, 35) days versus 22 (10, 52) days for the placebo group (p = 0.65). Mortality was comparable between the two groups (p > 0.05). Of the inflammatory markers, neutrophil counts were elevated in the vitamin C group relative to placebo by 72 h (p = 0.01). C-reactive protein and myeloperoxidase concentrations were elevated at baseline, however, the two groups were comparable over time (p > 0.05).
Conclusions: Our pilot study indicated that intravenous vitamin C did not provide significant decreases in the mean dose or duration of vasopressor infusion. Further research that takes into account the potential impact of intervention timing, dose and duration, and location of trial, may provide more definitive evidence.
Trial registration: ACTRN12617001184369 (11/8/2017).
Keywords: ICU length of stay; Noradrenaline; Sepsis; Septic shock; Vasopressor; Vitamin C.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Comment in
-
Vitamin C for septic shock in previous randomized trials: implications of erroneous dosing, timing, and duration.Crit Care. 2022 Mar 15;26(1):61. doi: 10.1186/s13054-022-03946-w. Crit Care. 2022. PMID: 35292098 Free PMC article. No abstract available.
-
Vitamin C therapy in septic shock.Crit Care. 2022 Mar 30;26(1):87. doi: 10.1186/s13054-022-03965-7. Crit Care. 2022. PMID: 35354493 Free PMC article. No abstract available.
References
-
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
-
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. - DOI - PubMed
-
- Fowler AA, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF, Gupta S, Fisher BJ, Natarajan R. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

