Elevated atmospheric CO2 alters the microbial community composition and metabolic potential to mineralize organic phosphorus in the rhizosphere of wheat
- PMID: 35074003
- PMCID: PMC8785599
- DOI: 10.1186/s40168-021-01203-w
Elevated atmospheric CO2 alters the microbial community composition and metabolic potential to mineralize organic phosphorus in the rhizosphere of wheat
Abstract
Background: Understanding how elevated atmospheric CO2 (eCO2) impacts on phosphorus (P) transformation in plant rhizosphere is critical for maintaining ecological sustainability in response to climate change, especially in agricultural systems where soil P availability is low.
Methods: This study used rhizoboxes to physically separate rhizosphere regions (plant root-soil interface) into 1.5-mm segments. Wheat plants were grown in rhizoboxes under eCO2 (800 ppm) and ambient CO2 (400 ppm) in two farming soils, Chromosol and Vertosol, supplemented with phytate (organic P). Photosynthetic carbon flow in the plant-soil continuum was traced with 13CO2 labeling. Amplicon sequencing was performed on the rhizosphere-associated microbial community in the root-growth zone, and 1.5 mm and 3 mm away from the root.
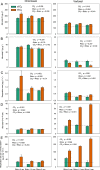
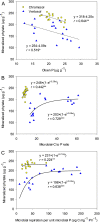
Results: Elevated CO2 accelerated the mineralization of phytate in the rhizosphere zones, which corresponded with increases in plant-derived 13C enrichment and the relative abundances of discreet phylogenetic clades containing Bacteroidetes and Gemmatimonadetes in the bacterial community, and Funneliformis affiliated to arbuscular mycorrhizas in the fungal community. Although the amplicon sequence variants (ASVs) associated the stimulation of phytate mineralization under eCO2 differed between the two soils, these ASVs belonged to the same phyla associated with phytase and phosphatase production. The symbiotic mycorrhizas in the rhizosphere of wheat under eCO2 benefited from increased plant C supply and increased P access from soil. Further supportive evidence was the eCO2-induced increase in the genetic pool expressing the pentose phosphate pathway, which is the central pathway for biosynthesis of RNA/DNA precursors.
Conclusions: The results suggested that an increased belowground carbon flow under eCO2 stimulated bacterial growth, changing community composition in favor of phylotypes capable of degrading aromatic P compounds. It is proposed that energy investments by bacteria into anabolic processes increase under eCO2 to level microbial P-use efficiencies and that synergies with symbiotic mycorrhizas further enhance the competition for and mineralization of organic P. Video Abstract.
Keywords: Climate change; Metabolic pathway; Microbial phosphorus; Microbial phylotypes; Phytate mineralization; Rhizobox.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Vitousek PM, Porder S, Houlton BZ, Chadwick OA. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl. 2010;20:5–15. - PubMed
-
- Gong H, Li Y, Yu T, Zhang S, Gao J, Zhang S, et al. Soil and climate effects on leaf nitrogen and phosphorus stoichiometry along elevational gradients. Glob Ecol Conserv. 2020;23:e01138.
-
- Rodriguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999;17:319–339. - PubMed
-
- Bergkemper F, Schöler A, Engel M, Lang F, Krüger J, Schloter M, et al. Phosphorus depletion in forest soils shapes bacterial communities towards phosphorus recycling systems. Environ Microbiol. 2016;18:1988–2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

