A sensitive and less cytotoxic assay for identification of proliferating T cells based on bioorthogonally-functionalized uridine analogue
- PMID: 35074315
- PMCID: PMC7614694
- DOI: 10.1016/j.jim.2022.113228
A sensitive and less cytotoxic assay for identification of proliferating T cells based on bioorthogonally-functionalized uridine analogue
Abstract
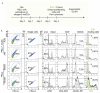
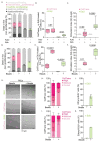
Quantitative detection of T cell proliferation is an important readout in immunology research, as it is one of the hallmarks of T cell activation. Fluorescence-based methods for T cell proliferation mostly rely on the usage of probes that non-specifically conjugate to free primary amine groups in cells. Each cell division then results in a two-fold dilution of the probes which is detectable with flow cytometry. However, questions have been raised about cytotoxicity of these dilution-based T cell proliferation probes and they potentially affect T cell activation. An alternative assay relies on the incorporation of the uridine analog BrdU in the DNA of dividing T cells that can be detected with an antibody, but this requires harsh fixation and denaturation conditions. Recently, a new assay for detection of cell proliferation has been developed, based on the incorporation of the bioorthogonally-functionalized uridine analog 5-ethynyl-2'-deoxyuridine (EdU). Goal of this study was to compare the sensitivity and cytotoxicity of the EdU assay with a widely-used dilution-based T cell proliferation probe, CellTrace Far Red. We found that, compared to the dilution-based probe, the EdU-based assay better preserves T cell viability, is more sensitive for detecting T cell proliferation, and allows for better discernable interferon gamma responses.
Keywords: Dilution-based probe; EdU; Ethynyl-uridine; T cell activation; T cell proliferation.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures



References
-
- Baranov MV, Bianchi F, Schirmacher A, van Aart MAC, Maassen S, Muntjewerff EM, Dingjan I, ter Beest M, Verdoes M, Keyser SGL, et al. The phosphoinositide kinase PIKfyve promotes Cathepsin-S-mediated major histocompatibility complex class II antigen presentation. IScience. 2019;11:160–177. - PMC - PubMed
-
- Cheraghali AM, Morin KW, Kumar R, Knaus EE, Wiebe LI. Accumulation of 5-ethyl-2’-deoxyuridine and its 5,6-dihydro prodrugs in murine lung and its potential clinical application. J Pharm Pharmacol. 1995;47:595–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

