The essential role for endothelial cell sprouting in coronary collateral growth
- PMID: 35074317
- PMCID: PMC8940680
- DOI: 10.1016/j.yjmcc.2022.01.005
The essential role for endothelial cell sprouting in coronary collateral growth
Abstract
Rationale: Coronary collateral growth is a natural bypass for ischemic heart diseases. It offers tremendous therapeutic benefit, but the process of coronary collateral growth isincompletely understood due to limited preclinical murine models that would enable interrogation of its mechanisms and processes via genetic modification and lineage tracing. Understanding the processes by which coronary collaterals develop can unlock new therapeutic strategies for ischemic heart disease.
Objective: To develop a murine model of coronary collateral growth by repetitive ischemia and investigate whether capillary endothelial cells could contribute to the coronary collateral formation in an adult mouse heart after repetitive ischemia by lineage tracing.
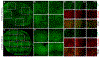
Methods and results: A murine model of coronary collateral growth was developed using short episodes of repetitive ischemia. Repetitive ischemia stimulation resulted in robust collateral growth in adult mouse hearts, validated by high-resolution micro-computed tomography. Repetitive ischemia-induced collateral formation compensated ischemia caused by occlusion of the left anterior descending artery. Cardiac function improved during ischemia after repetitive ischemia, suggesting the improvement of coronary blood flow. A capillary-specific Cre driver (Apln-CreER) was used for lineage tracing capillary endothelial cells. ROSA mT/mG reporter mice crossed with the Apln-CreER transgene mice underwent a 17 days' repetitive ischemia protocol for coronary collateral growth. Two-photon and confocal microscopy imaging of heart slices revealed repetitive ischemia-induced coronary collateral growth initiated from sprouting Apelin+ endothelial cells. Newly formed capillaries in the collateral-dependent zone expanded in diameter upon repetitive ischemia stimulation and arterialized with smooth muscle cell recruitment, forming mature coronary arteries. Notably, pre-existing coronary arteries and arterioles were not Apelin+, and all Apelin+ collaterals arose from sprouting capillaries. Cxcr4, Vegfr2, Jag1, Mcp1, and Hif1⍺ mRNA levels in the repetitive ischemia-induced hearts were also upregulated at the early stage of coronary collateral growth, suggesting angiogenic signaling pathways are activated for coronary collaterals formation during repetitive ischemia.
Conclusions: We developed a murine model of coronary collateral growth induced by repetitive ischemia. Our lineage tracing study shows that sprouting endothelial cells contribute to coronary collateral growth in adult mouse hearts. For the first time, sprouting angiogenesis is shown to give rise to mature coronary arteries in response to repetitive ischemia in the adult mouse hearts.
Keywords: Arterialization; Coronary circulation; Coronary collateral growth; Ischemic heart diseases; Postnatal coronary collateral formation; Repetitive ischemia.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors have no conflict of interest.
Figures

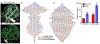






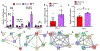
References
-
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al., Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association, Circulation 143(8) (2021) e254–e743. - PubMed
-
- Koerselman J, de Jaegere PP, Verhaar MC, Grobbee DE, der Graaf Y, Group SS, Cardiac ischemic score determines the presence of coronary collateral circulation, Cardiovasc Drugs Ther 19(4) (2005) 283–9. - PubMed
-
- Traupe T, Gloekler S, de Marchi SF, Werner GS, Seiler C, Assessment of the human coronary collateral circulation, Circulation 122(12) (2010) 1210–20. - PubMed
-
- Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C, The impact of the coronary collateral circulation on mortality: a meta-analysis, Eur Heart J 33(5) (2012) 614–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

