Recent advances in the mechanisms and treatment of immune thrombocytopenia
- PMID: 35074629
- PMCID: PMC8792416
- DOI: 10.1016/j.ebiom.2022.103820
Recent advances in the mechanisms and treatment of immune thrombocytopenia
Abstract
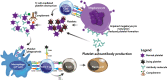
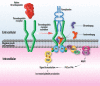
Primary immune thrombocytopenia is an autoimmune disease associated with a reduced peripheral blood platelet count. The phenotype is variable with some patients suffering no bleeding whilst others have severe bleeding which may be fatal. Variability in clinical behaviour and treatment responses reflects its complex underlying pathophysiology. Historically the management has relied heavily on immune suppression. Recent studies have shown that the older empirical immune suppressants fail to alter the natural history of the disease and are associated with a poor quality of life for patients. Newer treatments, such as the thrombopoietin receptor agonists, have transformed ITP care. They have high efficacy, are well tolerated and improve patients' quality of life. A greater understanding of the underlying pathophysiology of this disorder has helped develop a number of new targeted therapies. These include inhibitors of the neonatal Fc receptor inhibitors, Bruton tyrosine kinase and complement pathway. Here we discuss the mechanisms underlying ITP and the new approach to ITP care.
Keywords: immune suppression; immune thrombocytopenia; thrombopoietin receptor agonists.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests DP has received research support and honoraria from Amgen, Novartis, SOBI, UCB, argenx, Rigel and Chugai and has acted as a consultant for UCB, argenx, MedImmune and Ono; he also serves on a DSMB for an investigator-led study of rituximab in ITP and has received a basic shares package from previous employment by GlaxoSmithKline. JWS has received honoraria from Amgen, Novartis, and UCB and has acted as a consultant for Amgen, Novartis, Argenx and UCB.
Figures


References
-
- Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev. 2012;11:754–765. - PubMed
-
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. - PubMed
-
- Harrington WJ, Minnich V, Hollingsworth JW, Moore CV. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. Journal of Laboratory and Clinical Medicine. 1951;38:1–10. - PubMed
-
- Gernsheimer T, Stratton J, Ballem PJ, Slichter SJ. Mechanisms of response to treatment in autoimmune thrombocytopenic purpura. N Engl J Med. 1989;320:974–980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

