Convergent evolution of a blood-red nectar pigment in vertebrate-pollinated flowers
- PMID: 35074876
- PMCID: PMC8812537
- DOI: 10.1073/pnas.2114420119
Convergent evolution of a blood-red nectar pigment in vertebrate-pollinated flowers
Abstract
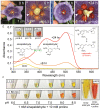
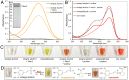
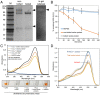
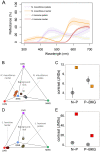
Nearly 90% of flowering plants depend on animals for reproduction. One of the main rewards plants offer to pollinators for visitation is nectar. Nesocodon mauritianus (Campanulaceae) produces a blood-red nectar that has been proposed to serve as a visual attractant for pollinator visitation. Here, we show that the nectar's red color is derived from a previously undescribed alkaloid termed nesocodin. The first nectar produced is acidic and pale yellow in color, but slowly becomes alkaline before taking on its characteristic red color. Three enzymes secreted into the nectar are either necessary or sufficient for pigment production, including a carbonic anhydrase that increases nectar pH, an aryl-alcohol oxidase that produces a pigment precursor, and a ferritin-like catalase that protects the pigment from degradation by hydrogen peroxide. Our findings demonstrate how these three enzymatic activities allow for the condensation of sinapaldehyde and proline to form a pigment with a stable imine bond. We subsequently verified that synthetic nesocodin is indeed attractive to Phelsuma geckos, the most likely pollinators of Nesocodon We also identify nesocodin in the red nectar of the distantly related and hummingbird-visited Jaltomata herrerae and provide molecular evidence for convergent evolution of this trait. This work cumulatively identifies a convergently evolved trait in two vertebrate-pollinated species, suggesting that the red pigment is selectively favored and that only a limited number of compounds are likely to underlie this type of adaptation.
Keywords: Jaltomata; Nesocodon; gecko; nectar; nectaries.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: A.D.H. and C.J.C. are named on a patent application (US2020/0474) by the University of Minnesota on the synthesis of the nesocodin pigment and associated non-natural derivatives.
Figures


References
-
- Cuthill I. C., et al. , The biology of color. Science 357, eaan0221 (2017). - PubMed
-
- Schaefer H. M., Schaefer V., Levey D. J., How plant-animal interactions signal new insights in communication. Trends Ecol. Evol. 19, 577–584 (2004).
-
- Moyroud E., Glover B. J., The evolution of diverse floral morphologies. Curr. Biol. 27, R941–R951 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

