Clinical and molecular characteristics associated with response to therapeutic PD-1/PD-L1 inhibition in advanced Merkel cell carcinoma
- PMID: 35074902
- PMCID: PMC8788332
- DOI: 10.1136/jitc-2021-003198
Clinical and molecular characteristics associated with response to therapeutic PD-1/PD-L1 inhibition in advanced Merkel cell carcinoma
Abstract
Background: Based on its viral-associated or UV-associated carcinogenesis, Merkel cell carcinoma (MCC) is a highly immunogenic skin cancer. Thus, clinically evident MCC occurs either in immuno-compromised patients or based on tumor-intrinsic immune escape mechanisms. This notion may explain that although advanced MCC can be effectively restrained by treatment with PD-1/PD-L1 immune checkpoint inhibitors (ICIs), a considerable percentage of patients does not benefit from ICI therapy. Biomarkers predicting ICI treatment response are currently not available.
Methods: The present multicenter retrospective study investigated clinical and molecular characteristics in 114 patients with unresectable MCC at baseline before treatment with ICI for their association with therapy response (best overall response, BOR). In a subset of 21 patients, pretreatment tumor tissue was analyzed for activation, differentiation and spatial distribution of tumor infiltrating lymphocytes (TIL).
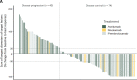
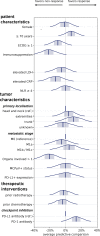
Results: Of the 114 patients, n=74 (65%) achieved disease control (BOR=complete response/partial response/stable disease) on ICI. A Bayesian cumulative ordinal regression model revealed absence of immunosuppression and a limited number of tumor-involved organ systems was highly associated with a favorable therapy response. Unimpaired overall performance status, high age, normal serum lactate dehydrogenase and normal serum C reactive protein were moderately associated with disease control. While neither tumor Merkel cell polyomavirus nor tumor PD-L1 status showed a correlation with therapy response, treatment with anti-PD-1 antibodies was associated with a higher probability of disease control than treatment with anti-PD-L1 antibodies. Multiplexed immunohistochemistry demonstrated the predominance of CD8+ effector and central memory T cells (TCM) in close proximity to tumor cells in patients with a favorable therapy response.
Conclusions: Our findings indicate the absence of immunosuppression, a limited number of tumor-affected organs, and a predominance of CD8+ TCM among TIL, as baseline parameters associated with a favorable response to PD-1/PD-L1 ICI therapy of advanced MCC. These factors should be considered when making treatment decisions in MCC patients.
Keywords: costimulatory and inhibitory T-cell receptors; immunotherapy; lymphocytes; skin neoplasms; translational medical research; tumor-Infiltrating.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SU declares research support from Bristol Myers Squibb and Merck Serono; speakers and advisory board honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Novartis and Roche, and travel support from Bristol Myers Squibb, and Merck Sharp & Dohme. LZ served as consultant and/or has received honoraria from Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre-Fabre, and Sanofi; Research funding to institution: Novartis; travel support from Merck Sharp & Dohme, Bristol-Myers Squibb, Amgen, Pierre-Fabre, and Novartis, outside the submitted work. PT declares invited speaker's honoraria from Bristol-Myers Squibb, Novartis, MSD, Pierre-Fabre, CureVac, Roche, Kyowa Kirin, Biofrontera, advisory board honoraria from Bristol-Myers Squibb, Novartis, Pierre-Fabre, Merck Serono, Sanofi, Roche, Kyowa Kirin, and travel support from Bristol-Myers Squibb, and Pierre-Fabre. UL declares advisory board honoraria from MSD, Roche, Sanofi, Novartis, Sun Pharma, Almirall Hermal. TE declares consulting fees from BMS, Novartis, Roche, Pierre Fabre, Sanofi; board membership and payment for lectures in speakers bureau from Pierre Fabre, MSD, Roche, BMS, Novartis and Sanofi. CL reports advisory board honoraria from MSD, BMS, Roche, Pierre Fabre, Novartis, Sun Pharma, Sanofi, Kyowa Kirin, Almiral Hermal, Biontech, Merc; speakers fee from MSD, BMS, Roche, Pierre Fabre, Novartis, Sun Pharma, Sanofi, Merck; travel reimbusment from MSD, BMS, Roche, Pierre Fabre, Novartis, Sun Pharma, Sanofi, Kyowa Kirin, Almiral Hermal, Biontech and Merck. JCH declares research support from Bristol Myers Squibb; advisory board honoraria from Pierre Fabre, Sanofi, Sun Pharma and Merck Sharp & Dome; speakers honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Roche, Sanofi and Almirall and travel support from Pierre Fabre. TG reports receiving speakers and/or advisory board honoraria from BMS, Sanofi-Grenzyme, MSD, Novartis Pharma, Roche, Abbvie, Almirall, Janssen Lilly, Pfizer, Pierre Fabre; speakers and/or advisory board honoraria from BMS, Sanofi-Grenzyme, MSD, Novartis, Pharma, Roche, Abbvie, Almirall, Janssen Lillly, Pfizer, Pierre Fabre. SH declares advisory boards honoraria from Pierre Fabre, MSD, BMS, Novartis, Sanofi. PM reports board membership and payment for lectures in speakers bureau from Pierre Fabre, GSK, MSD, Merck Germany, Roche, BMS, Novartis, Sanofi. P. Mohr reports research support (to institution): Bristol-MyersSquibb, Novartis, MSD. Honoraria for lectures (personally): Pierre Fabre, GSK, MSD, Merck Germany, Roche, BMS, Novartis, Sanofi, Amgen, SUN-Pharma, Roche Pharma, Bristol-MyersSquibb, Novartis, MSD, Almirall-Hermal, Amgen, Merck-Serono, Bayer, Pierre-Fabre, Sanofi. Honoraria for advisory boards: Pierre Fabre, GSK, MSD, Merck Germany, Roche, BMS, Novartis, Sanofi, Beiersdorf, Almiral-Hermal, AmgenBayersdorf, Roche Pharma, Bristol-MyersSquibb, Novartis, MSD, Almirall-Hermal, Amgen, Pierre-Fabre, Merck-Serono, SUN-Pharma, SUN, Merck-Serono, Sanofi. CP received honoraria (speaker honoraria or honoraria as a consultant) and travel support from Novartis, BMS, Roche, Merck Serono, MSD, Celgene, AbbVie, AMGEN, SUNPHARMA, Allergy Therapeutics and LEO. LMH served as consultant and/or has received honoraria from Amgen, BMS, Curevac, MSD, Novartis, Pierre-Fabre, Roche, Sanofi and Sun Pharma, outside the submitted work. Research funding to institution: Novartis. R. Gutzmer reports research support from Pfizer, Johnson & Johnson, Novartis, Amgen, MerckSerono, SUN Pharma; honoraria for lectures from Roche Pharma, Bristol-MyersSquibb, Novartis, MSD, Almirall-Hermal, Amgen, Merck-Serono, SUN, Pierre-Fabre, Sanofi, SUN Pharma, Bayer; honoraria for advice from Roche Pharma, Bristol-MyersSquibb, Novartis, MSD, Almirall-Hermal, Amgen, Pierre-Fabre, Merck-Serono, 4SC, Incyte, SUN Pharma, Sanofi, Pfizer. JSU is on the advisory board or has received honoraria and travel support from Amgen, Bristol Myers Squibb, GSK, LeoPharma, Merck Sharp and Dohme, Novartis, Pierre Fabre, Roche, Sanofi outside the submitted work. H-US is an employee of Targos Molecular Pathology Inc. and reports research support from Novartis Oncology and received honoraria from MSD, BMS, Roche Pharma, Novartis Oncology, AstraZeneca, Eisai, Takeda, Molecular Health, outside of the submitted work. DS reports personal fees from Amgen, GSK, BMS, Novartis, Roche, Merck, Astra Zeneca, Merck-Serono, Pfizer, Incyte, Array Pierre Fabre, Sanofi Genzyme, Regeneron, 4Sc, InFlaRx, Neracare, Ultimovacs, SunPharma, Philogen, Immunocore, Sandoz-Hexal outside the submitted work. JCB is receiving speaker's bureau honoraria from Amgen, Pfizer, MerckSerono, Recordati and Sanofi; is a paid consultant/advisory board member/DSMB member for Almirall, Boehringer Ingelheim, InProTher, ICON, MerckSerono, Pfizer, 4SC, and Sanofi/Regeneron. His group receives research grants from Bristol-Myers Squibb, Merck Serono, HTG, IQVIA, and Alcedis.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
