Antisense oligonucleotides as a potential treatment for brain deficits observed in myotonic dystrophy type 1
- PMID: 35075265
- PMCID: PMC9750879
- DOI: 10.1038/s41434-022-00316-7
Antisense oligonucleotides as a potential treatment for brain deficits observed in myotonic dystrophy type 1
Abstract
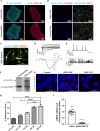
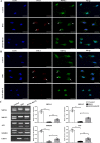
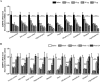
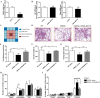
Myotonic dystrophy, or dystrophia myotonica type 1 (DM1), is a multi-systemic disorder and is the most common adult form of muscular dystrophy. It affects not only muscles but also many organs, including the brain. Cerebral impairments include cognitive deficits, daytime sleepiness, and loss of visuospatial and memory functions. The expression of mutated transcripts with CUG repeats results in a gain of toxic mRNA function. The antisense oligonucleotide (ASO) strategy to treat DM1 brain deficits is limited by the fact that ASOs do not cross the blood-brain barrier after systemic administration, indicating that other methods of delivery should be considered. ASO technology has emerged as a powerful tool for developing potential new therapies for a wide variety of human diseases, and its potential has been proven in a recent clinical trial. Targeting DMPK mRNA in neural cells derived from human induced pluripotent stem cells obtained from a DM1 patient with the IONIS 486178 ASO abolished CUG-expanded foci, enabled nuclear redistribution of MBNL1/2, and corrected aberrant splicing. Intracerebroventricular injection of the IONIS 486178 ASO in DMSXL mice decreased the levels of mutant DMPK mRNAs by up to 70% throughout different brain regions. It also reversed behavioral abnormalities following neonatal administration. The present study indicated that the IONIS 486178 ASO targets mutant DMPK mRNAs in the brain and strongly supports the feasibility of a therapy for DM1 patients based on the intrathecal injection of an ASO.
© 2022. The Author(s).
Conflict of interest statement
KKL, CFB, and FR are employees of Ionis Pharmaceuticals. The remaining authors declare no competing interests.
Figures


Similar articles
-
Enhanced Delivery of Ligand-Conjugated Antisense Oligonucleotides (C16-HA-ASO) Targeting Dystrophia Myotonica Protein Kinase Transcripts for the Treatment of Myotonic Dystrophy Type 1.Hum Gene Ther. 2022 Aug;33(15-16):810-820. doi: 10.1089/hum.2022.069. Hum Gene Ther. 2022. PMID: 35794764
-
Systemic therapy in an RNA toxicity mouse model with an antisense oligonucleotide therapy targeting a non-CUG sequence within the DMPK 3'UTR RNA.Hum Mol Genet. 2020 Jun 3;29(9):1440-1453. doi: 10.1093/hmg/ddaa060. Hum Mol Genet. 2020. PMID: 32242217 Free PMC article.
-
Recent Progress and Challenges in the Development of Antisense Therapies for Myotonic Dystrophy Type 1.Int J Mol Sci. 2022 Nov 1;23(21):13359. doi: 10.3390/ijms232113359. Int J Mol Sci. 2022. PMID: 36362145 Free PMC article. Review.
-
Short antisense-locked nucleic acids (all-LNAs) correct alternative splicing abnormalities in myotonic dystrophy.Nucleic Acids Res. 2015 Mar 31;43(6):3318-31. doi: 10.1093/nar/gkv163. Epub 2015 Mar 9. Nucleic Acids Res. 2015. PMID: 25753670 Free PMC article.
-
Antisense oligonucleotides: rising stars in eliminating RNA toxicity in myotonic dystrophy.Hum Gene Ther. 2013 May;24(5):499-507. doi: 10.1089/hum.2012.212. Epub 2013 Jan 30. Hum Gene Ther. 2013. PMID: 23252746 Free PMC article. Review.
Cited by
-
Muscle-specific gene editing improves molecular and phenotypic defects in a mouse model of myotonic dystrophy type 1.Clin Transl Med. 2025 Feb;15(2):e70227. doi: 10.1002/ctm2.70227. Clin Transl Med. 2025. PMID: 39956955 Free PMC article.
-
Pluripotent Stem Cells in Disease Modeling and Drug Discovery for Myotonic Dystrophy Type 1.Cells. 2023 Feb 10;12(4):571. doi: 10.3390/cells12040571. Cells. 2023. PMID: 36831237 Free PMC article. Review.
-
Localization of unlabeled bepirovirsen antisense oligonucleotide in murine tissues using in situ hybridization and CARS imaging.RNA. 2023 Oct;29(10):1575-1590. doi: 10.1261/rna.079699.123. Epub 2023 Jul 17. RNA. 2023. PMID: 37460153 Free PMC article.
-
The Coming Age of Antisense Oligos for the Treatment of Hepatic Ischemia/Reperfusion (IRI) and Other Liver Disorders: Role of Oxidative Stress and Potential Antioxidant Effect.Antioxidants (Basel). 2024 May 31;13(6):678. doi: 10.3390/antiox13060678. Antioxidants (Basel). 2024. PMID: 38929116 Free PMC article. Review.
-
The use of RNA-based treatments in the field of cancer immunotherapy.Mol Cancer. 2023 Jul 7;22(1):106. doi: 10.1186/s12943-023-01807-w. Mol Cancer. 2023. PMID: 37420174 Free PMC article. Review.
References
-
- PS H. Myotonic Dystrophy. London: WB Saunders; 2001.
-
- Bassez G, Lazarus A, Desguerre I, Varin J, Laforet P, Becane HM, et al. Severe cardiac arrhythmias in young patients with myotonic dystrophy type 1. Neurology. 2004;63:1939–41. doi: 10.1212/01.WNL.0000144343.91136.CF. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

