This is a preprint.
Mucociliary Transport Deficiency and Disease Progression in Syrian Hamsters with SARS-CoV-2 Infection
- PMID: 35075457
- PMCID: PMC8786228
- DOI: 10.1101/2022.01.16.476016
Mucociliary Transport Deficiency and Disease Progression in Syrian Hamsters with SARS-CoV-2 Infection
Update in
-
Mucociliary transport deficiency and disease progression in Syrian hamsters with SARS-CoV-2 infection.JCI Insight. 2023 Jan 10;8(1):e163962. doi: 10.1172/jci.insight.163962. JCI Insight. 2023. PMID: 36625345 Free PMC article.
Abstract
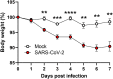
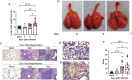
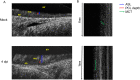
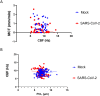
Substantial clinical evidence supports the notion that ciliary function in the airways plays an important role in COVID-19 pathogenesis. Although ciliary damage has been observed in both in vitro and in vivo models, consequent impaired mucociliary transport (MCT) remains unknown for the intact MCT apparatus from an in vivo model of disease. Using golden Syrian hamsters, a common animal model that recapitulates human COVID-19, we quantitatively followed the time course of physiological, virological, and pathological changes upon SARS-CoV-2 infection, as well as the deficiency of the MCT apparatus using micro-optical coherence tomography, a novel method to visualize and simultaneously quantitate multiple aspects of the functional microanatomy of intact airways. Corresponding to progressive weight loss up to 7 days post-infection (dpi), viral detection and histopathological analysis in both the trachea and lung revealed steadily descending infection from the upper airways, as the main target of viral invasion, to lower airways and parenchymal lung, which are likely injured through indirect mechanisms. SARS-CoV-2 infection caused a 67% decrease in MCT rate as early as 2 dpi, largely due to diminished motile ciliation coverage, but not airway surface liquid depth, periciliary liquid depth, or cilia beat frequency of residual motile cilia. Further analysis indicated that the fewer motile cilia combined with abnormal ciliary motion of residual cilia contributed to the delayed MCT. The time course of physiological, virological, and pathological progression suggest that functional deficits of the MCT apparatus predispose to COVID-19 pathogenesis by extending viral retention and may be a risk factor for secondary infection. As a consequence, therapies directed towards the MCT apparatus deserve further investigation as a treatment modality.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
